Fig. 27.1
Schloffer’s transnasal transsphenoidal operation. (a) Incision made along the left nasolabial furrow around the left ala nasi and continued up to the glabella. (b) The incision cuts through the skin, nasal bone, philtrum, and the anterior part of the septum. The whole external nose is reflected to the right, exposing the remainder of the septum. (c) The rest of the nasal septum has been removed, exposing the rostrum of the sphenoid sinus. (d) The anterior wall of the sphenoid sinus is opened, the mucosa lining of the sinus is removed with a sharp spoon, and the floor of the sella is removed with a small chisel or punch forceps. (From: Cope VZ: The pituitary fossa, and the methods of surgical approach thereto. Br J Surg 4:107–144, 1916)
Indications for Surgery
The gold standard for treating both symptomatic extra-glandular nonsecreting and glandular secreting pituitary adenomas, with the exception of prolactin-secreting tumors, is surgical resection. A trial of medical therapy with octreotide or cabergoline for growth hormone (GH)-secreting tumors may be warranted (especially in GH–prolactin (PRL)-cosecreting tumors). Ketoconazole may be used for adrenocorticotropic hormone (ACTH)-secreting tumors as an initial mode of therapy in patients with multiple comorbidities or in those patients who are reluctant to undergo surgery [8–12]. In patients who fail to respond to medical therapy or those who experience intolerable side effects, surgical resection becomes necessary. In certain instances of progressive visual loss caused by increasing mass effect or in cases of sudden onset of visual loss or ophthalmoplegia associated with severe headache and acute adrenal insufficiency as with pituitary apoplexy, emergent surgical decompression of the optic apparatus along with steroid replacement is warranted [13–16]. The general goals of surgery are removal of the tumor mass with decompression of the optic apparatus, normalization of hormonal hypersecretion, and preservation of normal pituitary function (Fig. 27.2). Radiation therapy, either fractionated radiosurgery or gamma knife surgery, may be used as part of a planned combination therapy for large tumors extending into the cavernous sinus with internal carotid artery encasement, as a salvage treatment for recurrent tumor, or as the primary mode of treatment for patients who are poor surgical candidates [17–20].
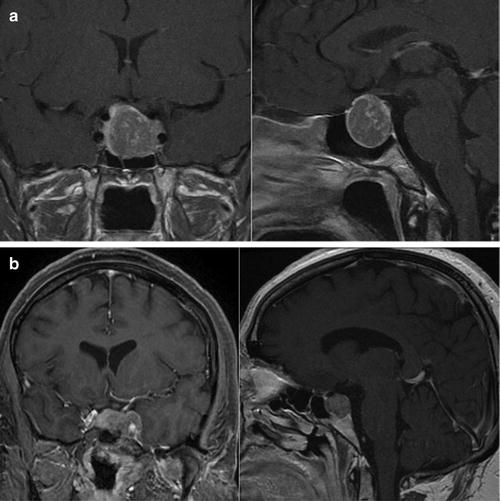

Fig. 27.2
(a) MR images of the brain with gadolinium enhancement demonstrating a large sellar and suprasellar GH-secreting pituitary macroadenoma in a 36-year-old acromegalic woman who presented with a 4-year history of amenorrhea and a GH level of 60 ng/mL. Gross total resection was achieved via a transnasal transsphenoidal resection.(b) MR images of the brain with gadolinium enhancement showing a sellar ACTH-secreting pituitary adenoma with extension into the region of the left cavernous sinus in a 38-year-old Cushingoid woman who presented with an elevated fasting serum cortisol level of 99 μg/dL and significantly elevated 24-h urinary cortisol. The patient underwent a microscopic transsphenoidal resection of her tumor with endoscopic assistance. A cure was achieved with a drop in her postoperative cortisol level to 1.7 μg/dL. She was subsequently placed on hormone replacement therapy
Endocrinologic, Anesthetic, and Radiographic Considerations and Planning
The radiographic features of the pituitary tumor and its associated endocrinopathies have important medical, anesthetic, and surgical implications. Before considering surgical intervention, control and medical management of any preexisting medical conditions and endocrinologic abnormalities should be instituted. This is best accomplished in an interdisciplinary manner with the help of an endocrinologist. Hyperthyroidism should be treated with thyroxine, with the aim of reducing cardiac and sympathetic overactivity. Elevated blood sugar levels caused by GH- and ACTH-secreting tumors should be managed. Conversely, patients with hypoactive pituitary function should receive appropriate replacement therapy to reestablish homeostasis before undergoing surgery [12, 21, 22]. All patients should have baseline fasting pituitary laboratory measurements including prolactin, thyroid-stimulating hormone (TSH), free T4, ACTH, and fasting AM cortisol; follicle-stimulating hormone, luteinizing hormone, GH, and insulin-like growth factor (IGF-1); and testosterone (in males) and estradiol (in females) levels [11, 23, 24]. Patients with visual disturbance should undergo a formal baseline visual field and acuity testing by a neuro-ophthalmologist [25]. Special attention should be paid to thyroid and steroid replacement; low levels of these hormones may result in increased surgical and perioperative risk, and they should be replaced accordingly. Hypocortisolemia may be replaced acutely with intravenous corticosteroids, but thyroid hormone deficit may take several days to replace.
In planning for surgery, the anesthesiologist should be cognizant of the medical and physical stigmata of the pituitary tumor–related endocrinopathies because they have important implications with regard to preoperative care and readiness. Patients on bromocriptine therapy may suffer from gastroparesis and have occasional vomiting [16, 23, 26]. Such patients should be appropriately identified and suitable measures taken to control their nausea and prevent aspiration. Acromegalic patients should be evaluated for sleep apnea, snoring, and airway changes prior to intubation [27–29]. In addition, electrocardiography and a transthoracic echocardiogram to evaluate for baseline left ventricular function are warranted since patients with acromegaly are at risk for hypertrophic cardiomyopathy [30, 31]. If an arterial line is required throughout the case, especially in patients with known hypertension, some anesthesiologists will advocate placing the line in the dorsalis pedis artery since acromegalic patients may have an impaired ulnar artery circulation. In the event that a radial arterial line is contemplated, an Allen test should be performed to document good collateral flow [32]. Cushingoid patients with hypercortisolemia are easily bruised, and their peripheral veins can be difficult to cannulate [16]. Special care in both positioning and venous access should be practiced in such patients. Intravenous hydrocortisone (100 mg) may be given at the start of the procedure to prevent diabetes insipidus resulting from antidiuretic hormone deficiency [23, 24, 33]. The anesthesiologist also needs to be mindful of the very short closure time involved at the conclusion of the resection to adjust the anesthetic dose accordingly in preparation for awakening. In the rare instance that air is to be injected through a lumbar drain to elevate the cerebrospinal fluid pressure and aid in the delivery and resection of a suprasellar component of the tumor (a practice that is not employed at our facility), the anesthesiologist should eliminate nitrous oxide from the anesthetic gas mixture to prevent tension pneumocephalus [34].
The surgical route of choice is best determined by careful inspection and evaluation of the morphology of the tumor of interest and the area it occupies on thin-sliced coronal and sagittal magnetic resonance images through the sellar region while considering ease of access and patient safety. The vast majority of pituitary tumors are removed via the transsphenoidal approach. Lesions confined to the sella without any suprasellar extension can be easily resected via a transsphenoidal route. Despite the suprasellar extension of some sellar lesions, however, transsphenoidal surgery may still be used as long as the tumor appears to be centrally located and symmetric. The “hour-glass” or “dumb-bell” appearance of a tumor may necessitate a combined approach for maximal safe resection, if the tumor does not descend from below [35–38]. Asymmetric extension of the tumor either anteriorly or posteriorly may pose a challenge for the surgeon attempting to tackle it via a transsphenoidal approach but may be amenable to transsphenoidal resection with wider removal of the skull base for exposure. Some lesions are fibrous in consistency, making them difficult to resect through a transsphenoidal route, especially in cases where the tumor may be adherent to important nearby neurovascular structures, or masking important arterial feeders such as the superior hypophyseal artery supplying the optic chiasm [35, 37, 39–41]. Another important consideration is the proximity of the tumor to the intracavernous carotid arteries. This is particularly important for identifying deviations of the eccentric carotid arteries, which may deviate into a midline trajectory and preclude a transsphenoidal approach [37]. Fine-cut computed tomography (CT) of the head with three-dimensional reconstruction helps identify important bony landmarks, demonstrate the extent of sellar expansion, and elucidate any evidence of tumor erosion through the sellar wall. This becomes increasingly important in repeat transsphenoidal surgeries in which the normal anatomy is perturbed and may therefore be misleading [37, 42]. In such a scenario, a stereotactic CT scan is useful for stereotactic neuronavigation, which helps to confirm midline and avoid inadvertent injury to the carotid or violation of the anterior fossa space.
Surgical Approaches to the Pituitary
The Direct Microscopic Endonasal Transsphenoidal Approach
Hirsch was the first to adopt the endonasal route to the sphenoid sinus using multiple-staged operations including resection of the middle turbinate and ethmoidectomy [43]. Despite his success, however, he changed his technique to a transseptal approach. Almost eight decades later, in 1987, Griffith and Veerapen revived the endonasal approach to the sphenoid sinus [44].
The patient is placed in the supine “reflex” position with the knee and body slightly bent at the mid-position to prevent entrapment neuropathy, venous pooling, and patient slippage (Fig. 27.3). The head is slightly tilted to the contralateral side, the neck is flexed, and the head is extended at the occipitocervical joint and slightly turned toward the surgeon. This ensures that the face is parallel to the floor and places the sphenoid ostia in the direct line of sight of the surgeon. The head may be fixed in a three-point Mayfield frame or on a horseshoe head holder and secured with tape. At least 10 min prior to incision, the nostrils are packed with oxymetazoline-soaked cottonoids to allow shrinkage of the turbinates and to optimize hemostasis during initial exposure. We no longer routinely prep the nose. The ipsilateral thigh or abdomen is prepped and draped for harvest of fat and fascia. Some surgeons use intraoperative C-arm fluoroscopy for guidance; however, we prefer to use such assistance only in selected cases. The rostrum of the sphenoid is identified at the level of the superior aspect of the distal middle turbinate with direct vision using a handheld speculum. The sphenoid ostium is found as the superior landmark of the opening of the rostrum just at the level of the inferior border of the superior turbinate. The perpendicular plate of the ethmoid is fractured at the junction of the sphenoid rostrum. A self-retaining nasal retractor is placed with the remnant of the perpendicular plate of the ethmoid in the midline. The mucosa are elevated away from each side of the sphenoid rostrum. The sphenoid ostia should become visible, and the sphenoid rostrum is removed with the aid of micro rongeurs or a high-speed drill. The sella is identified, and the bone over the sella is removed with micro rongeurs. A #11 blade is used to create a durotomy in an X-fashion, and then small, angled microscissors are used to further open the dura to the extremes of exposure desired (Fig. 27.4). With large tumors, the intracranial pressure helps deliver the tumor when the dura is opened. First, the floor of the tumor is removed with curettes. Then, the lateral aspects of the tumor are removed to enable descent of the large center part of the tumor. Four-quadrant curettage with various Hardy ring curettes allows further tumor removal from lateral, superior, and inferior recesses. Closure begins with packing the resection site with adipose tissue. The septum is reduced to the midline, and the authors elevate the middle turbinates on both sides to ensure unimpeded drainage of the maxillary sinuses. The authors employ well-lubricated, soft, curved Rhino Rocket tubes covered with a piece of latex as airway tubes, which are carefully placed posteriorly into the nasopharynx [45, 46].
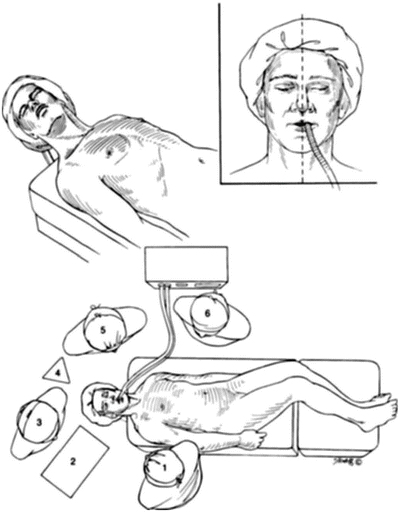
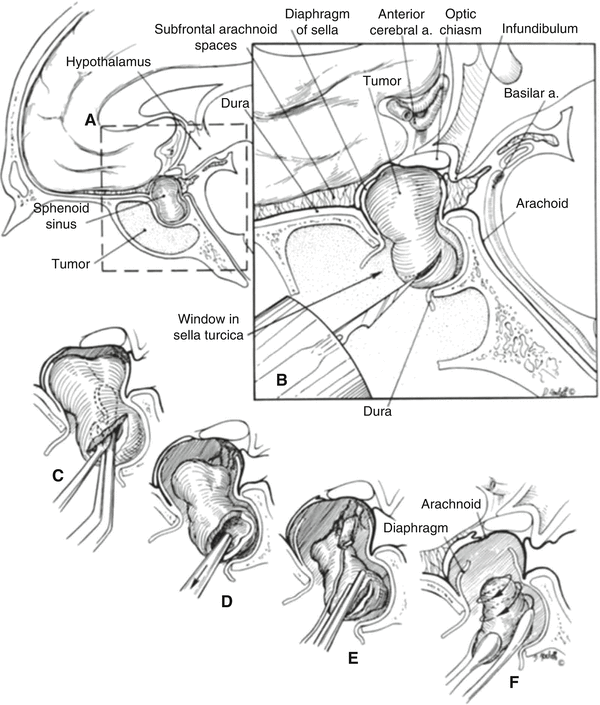

Fig. 27.3
Patient and operating room positioning for the transsphenoidal approach. The head is elevated 15° above to reduce venous bleeding. The head is tilted toward the left shoulder to facilitate endonasal exposure. (From: Couldwell WT, Weiss MH. The transnasal transsphenoidal approach. In: Apuzzo MLJ, ed. Surgery of the third ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998. p. 553–74, with permission)

Fig. 27.4
Tumor removal. Tumor is readily removed with various curettes and scoops. It is imperative that the integrity of the arachnoid is preserved to minimize the potential for a postoperative cerebrospinal fluid fistula. (From: Couldwell WT, Weiss MH. The transnasal transsphenoidal approach. In: Apuzzo MLJ, ed. Surgery of the third ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998. p. 553–74, with permission)
Advancements in Endoscopic Surgery
The successful reintroduction of the pure endonasal route paved the way for the use of endoscopic surgery in addressing sellar and suprasellar lesions [47, 48]. There are many advantages to the employment of endoscopy as a sole or supplemental tool for the resection of pituitary lesions. The endoscope provides a panoramic view of the sphenoid sinus and its important bony impressions. It also affords the surgeon with the ability to zoom in on areas of interest such as the tumor–gland interface and obtain a sharpened close-up view. Lenses of various angles on the endoscope allow the operator to look into anatomic corners of the suprasellar region that otherwise cannot be seen with the conventional microscope. Additionally, there is no displacement of the midline septum, and nasal packing and retraction are not needed [49–52]. Notwithstanding the improved and versatile visibility afforded by endoscopic transsphenoidal surgery, the technique is not without pitfalls. In general, more of the nasal septum is resected to allow adequate visual access. With endoscopic surgery, the surgeon’s field of view is reflected on a screen as a two-dimensional image, which results in loss of the sharp and focused stereoscopic view and depth perception provided by the microscope. In addition, there is a steep learning curve for the neurosurgeon inexperienced in endoscopy [53–55]. In our hybrid practice, we have been increasingly employing the endoscope as a supplemental tool for inspecting areas that are hidden from the field of view of the microscope in all of skull base surgery. In lateral and extreme anterior and posterior tumor rests, and those that are buried behind redundant folds of suprasellar arachnoid as well as in cases of tumor extending into the cavernous sinus, the endoscope is a valuable tool.
Modifications of the Transsphenoidal Approach
The standard transsphenoidal approach is used in most cases of pituitary tumors with sellar and suprasellar extension; however, regions of the skull base that were once thought only accessible “from above” are now being approached transfacially. With better knowledge of microsurgical anatomy and modern microinstrumentation, neurosurgeons have modified the transsphenoidal approach to gain better access to regions such as the cavernous sinus and the suprasellar cisterns.
Fraioli et al. [56] treated 11 patients with sellar tumors invading the medial wall of the cavernous sinus with a transmaxillosphenoidal approach, which involves a unilateral, bilateral, or Le Fort maxillary osteotomy and removal of the medial wall of the maxillary sinus in addition to the standard transsphenoidal exposure. It allows direct visualization of the intracavernous carotid artery during tumor resection but is limited by extension of tumor lateral to the carotid artery. Sabit et al. [57] described a safe, minimally invasive combined transmaxillary transsphenoidal approach to the cavernous sinus that is both extradural and extranasal. This approach provides adequate lateral-to-medial reach in the parasellar and infrasellar regions with visualization of the entire ipsilateral cavernous sinus and the medial aspect of the contralateral cavernous sinus. The extended transsphenoidal approach to tumors of the tuberculum sellae and suprasellar cistern was first described by Weiss [58]. Suprasellar tumors without sellar enlargement have been successfully resected using a modified transsphenoidal approach that involves a wide bone exposure of the anterior surface of the sella and removal of the posterior portion of the planum sphenoidale [59, 60]. This novel approach has allowed resection of craniopharyngiomas, central nervous system hemangioblastomas, and ectopic ACTH-producing adenomas arising from the pituitary stalk [59, 60].
Additional exposure of the skull base for lesions of the parasellar and clival region can be achieved by extended transsphenoidal approaches [36]. Various portions of the skull base may be exposed, and bone resection can be extended by repositioning the patient’s head and the self-retaining speculum. The standard transsphenoidal approach can be extended anteriorly to resect suprasellar lesions, inferiorly to expose clival lesions, and inferolaterally to access cavernous sinus lesions (Figs. 27.5, 27.6, and 27.7) [36]. These variations on the transsphenoidal approach provide a minimally invasive technique that avoids prolonged surgery and brain retraction.
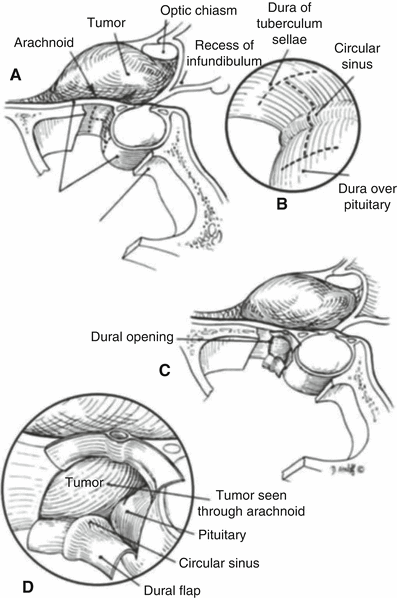
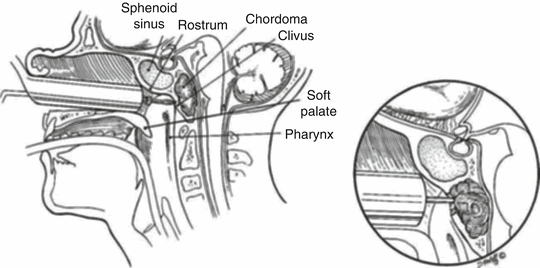
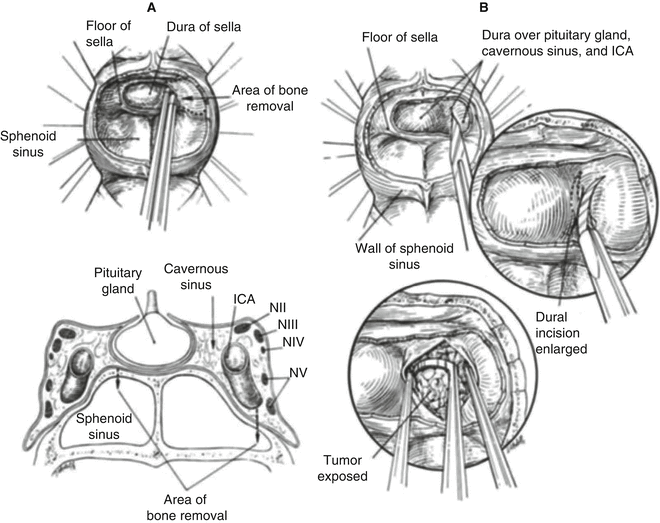

Fig. 27.5
(a–d) Modification of the transsphenoidal approach: exposure of the anterior skull base (extended transsphenoidal). A pure suprasellar tumor may be approached by extending the bony resection anteriorly over the tuberculum sellae, thus exposing the dura mater lying anterior to the circular sinus. An incision is made in the dura anteriorly and inferiorly to the circular sinus. The sinus is then coagulated and transected to gain a direct view of the suprasellar cistern without disturbing the pituitary gland. (From: Couldwell WT, Weiss MH. The transnasal transsphenoidal approach. In: Apuzzo MLJ, ed. Surgery of the third ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998. p. 553–74, with permission)

Fig. 27.6
Modifications of the transsphenoidal approach: inferior exposure of the clivus. Exposure of the clivus is facilitated by slight flexion of the patient’s head and repositioning of the nasal self-retaining retractor to point inferiorly. The upper clivus lies directly posterior to the sphenoid sinus, but additional exposure to the mid and lower clivus requires more inferior exposure. (From: Couldwell WT, Weiss MH. The transnasal transsphenoidal approach. In: Apuzzo MLJ, ed. Surgery of the third ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998. p. 553–74, with permission)

Fig. 27.7
Modification of the transsphenoidal approach: inferolateral exposure of the cavernous sinus. (a) After exposure of the dura overlying the sella, the bone overlying the cavernous sinus, including that overlying the carotid grooves, is carefully removed. This removal defines the lateral extent of the exposure limited by the cavernous cranial nerves. (b) The dura medial to the internal carotid artery is first incised with a size 11 blade and opened with curved alligator microscissors. Removal of the intracavernous portion of the tumor is carried out with a microcurette. (From: Couldwell WT, Weiss MH. The transnasal transsphenoidal approach. In: Apuzzo MLJ, ed. Surgery of the third ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998. p. 553–74, with permission)
Technological advances in the areas of endoscope-assisted microneurosurgery [47, 49] have been applied to the classical transsphenoidal operation in an attempt to further decrease morbidity and mortality risks [47, 52, 55]. Jho and Carrau [52] reported encouraging results in a series of 50 patients who underwent endoscopic endonasal transsphenoidal surgery. One of the main advantages of this approach is excellent panoramic visualization of the sellar and suprasellar anatomy with increased illumination and magnification [48]. Anatomic studies have demonstrated that the endoscope provides a volume of exposure superior to that of the operating microscope [54], but disadvantages include the lack of stereoscopic vision, the lack of adequate instrumentation, the limited space of working through one nostril [49], and a learning curve for using this technique.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree