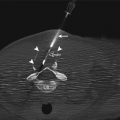
Fig. 12.1
US-guided pleural biopsy. Axial CT image of the chest (a) shows a focal pleural mass (arrow). Grayscale ultrasound image (b) during the biopsy shows the echogenic biopsy needle (arrow) within the mass (M). The thin echogenic band (arrowheads) signifies the interface of the mass (M) and the underlying lung tissue (Ahrar et al. [13])
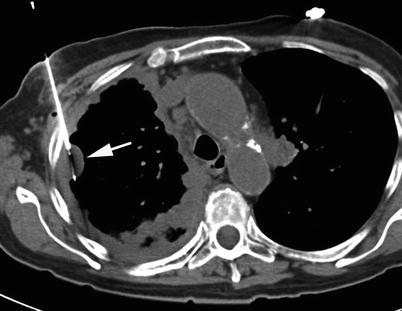
Fig. 12.2
CT-guided pleural biopsy. Axial CT image shows diffuse right pleural thickening with a focal nodular mass (arrow). A core biopsy needle is placed within the pleural mass (Ahrar et al. [13])
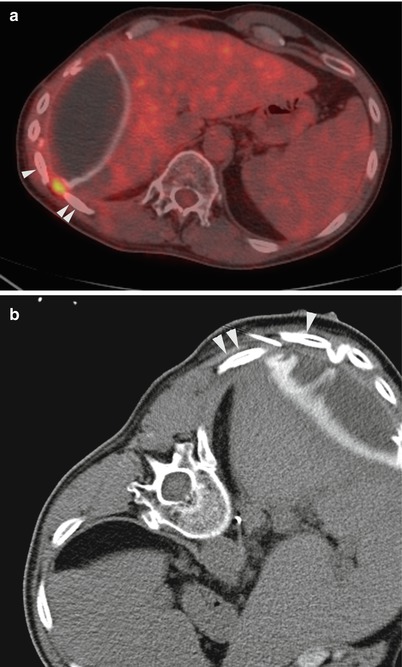
Fig. 12.3
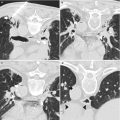
CT-guided pleural biopsy with minimal pleural thickening. A 53-year-old man with history of epithelioid malignant mesothelioma and extrapleural pneumonectomy underwent PET-CT imaging for surveillance. Fused axial PET-CT image (a) showed a focal area of FDG uptake in the posterolateral chest wall between the right 10th (arrowhead) and the 11th (double arrowhead) ribs. Minimal pleural thickening was targeted in that particular interspace under CT guidance (b). Pathology confirmed recurrent malignant mesothelioma
Contraindications
The only contraindication for image-guided pleural biopsy is the presence of uncorrectable coagulopathy. Some researchers have suggested that pleural biopsy is contraindicated in patients who cannot control their breathing or who cannot cooperate during the biopsy [16]. In our experience, most patients under moderate sedation take regular, shallow breaths that help target even small pleural nodules.
Pleural Biopsy Technique
Overview
Surgical pleural biopsy, also referred to as “open” pleural biopsy, is the “gold standard” for diagnosis of pleural diseases, but thoracotomy is associated with substantial morbidity [17–20]. Minimally invasive surgical approaches to pleural biopsy, including video-assisted thoracoscopic surgery (VATS) and physician-based thoracoscopy, have also demonstrated a high diagnostic yield (>90 %) [21–26]. However, VATS and thoracoscopy require a safe separation of the visceral and parietal pleura, and medical thoracoscopy requires the presence of a moderate effusion [27], whereas image-guided pleural biopsy is not limited by these factors.
Nonsurgical percutaneous pleural biopsy, also known as “blind” or “closed” pleural biopsy, was first described in patients with pleural effusion in the 1950s by Abrams and Cope [28, 29]. In this technique, a reverse-beveled needle is advanced into the pleural cavity and pulled back to “hook” the pleura and obtain a biopsy sample (Fig. 12.4). Four to six passes are usually necessary to obtain an adequate diagnostic specimen [8]. Although blind pleural biopsy can be performed at the bedside and is relatively simple, the diagnostic yield is suboptimal and the complication rates are higher than those for image-guided biopsy [30]. Simultaneous fluid analysis with blind pleural biopsy has demonstrated better diagnostic value than blind biopsy alone [31].
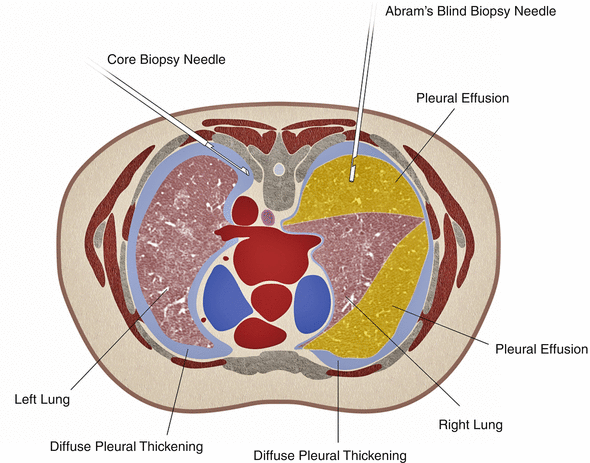

Fig. 12.4
Artist rendition of bilateral diffuse pleural thickening. With the patient in prone position, the right pleura can be sampled by blind biopsy (e.g., with Abrams needle) due to presence of large pleural effusion. On the left side, the lack of pleural effusion precludes blind pleural biopsy. A core biopsy needle is shown in the pleural thickening on the left
Over the last two decades, the trend has been away from blind pleural biopsy and toward image-guided pleural biopsy, using either ultrasonography or computed tomography (CT) [32].
Imaging
Both CT and ultrasonography can be used to guide pleural biopsies. Initially, a diagnostic imaging study, usually a CT (Fig. 12.5) scan or a PET-CT (Fig. 12.3) scan, identifies areas of nodular, patchy, or diffuse pleural thickening. Subsequently, the choice between CT and ultrasound depends on personal preference, local expertise, and equipment availability. Ultrasonography provides real-time imaging, is widely available, and is relatively inexpensive. However, pleural lesions near or behind the ribs or along the paravertebral or medial surfaces may be difficult to image with ultrasound, and small pleural nodules in the absence of pleural effusion may be challenging to localize with ultrasound (Fig. 12.6). CT can help identify and target virtually any nodule or area of pleural thickening in the thoracic cavity.
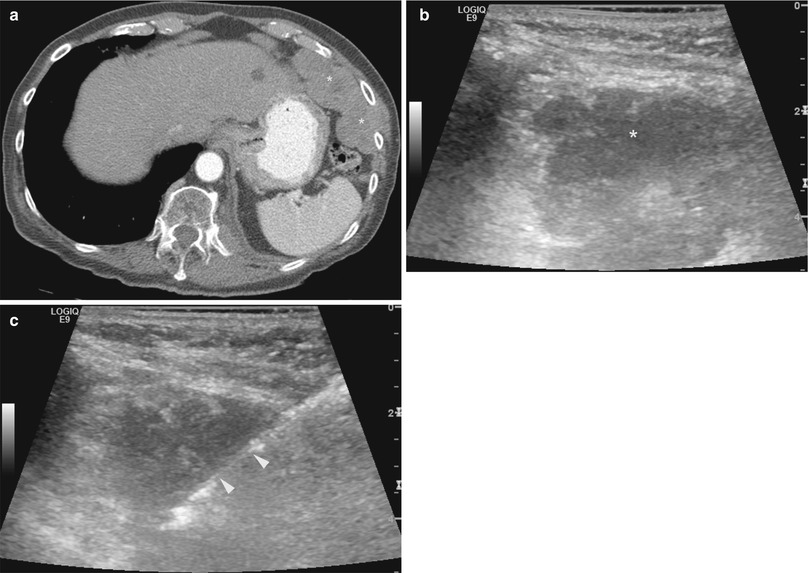
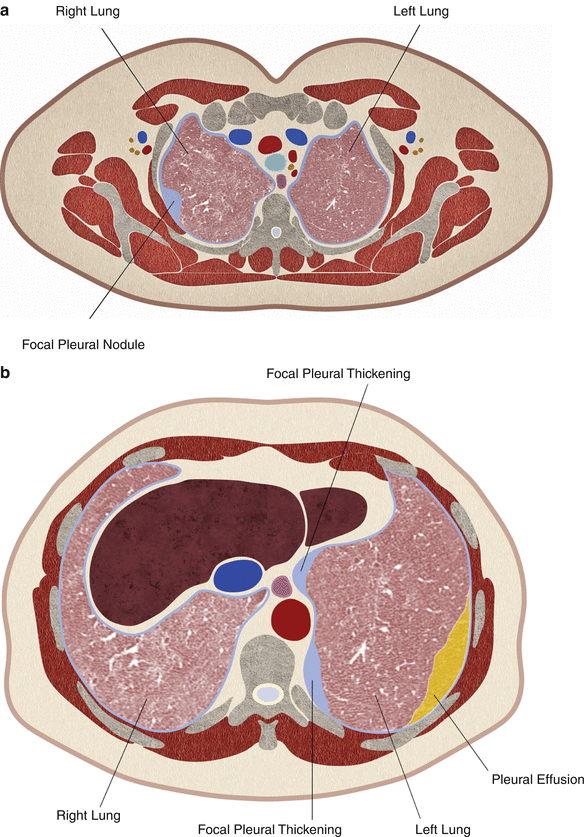

Fig. 12.5
Ultrasound-guided biopsy of pleural mass extending into the chest wall. An 84-year-old man with new onset of pain underwent CT imaging which showed multiple pleural masses involving the left hemithorax (a). The largest mass was found in the anterolateral aspect of the left lower chest wall. Grayscale ultrasound image of the chest wall (b) shows the hypoechoic pleural mass. Biopsy was carried out under ultrasound guidance (c). Arrowheads indicate the cutting tip of a core biopsy needle. Pathology showed high-grade carcinoma of unknown primary

Fig. 12.6
Artist rendition of focal pleural nodules along the superior-lateral aspect of the right pleural cavity (a) and along the inferior-medial aspect of the left pleural cavity (b). CT guidance is preferred for targeting these small nodules that are difficult to visualize with ultrasound
Devices
For blind pleural biopsy, Abrams or Cope needles are used. These needles range in size from 11 to 8 gauge (up to 4 mm in diameter). Each needle is fitted with a “cutting window” to trap and extract a sample of the pleura. Safe application of these devices requires the presence of a moderate-sized pleural effusion (Fig. 12.4).
For image-guided biopsies, both fine-needle aspiration (FNA) biopsy (Fig. 12.7) and automated tissue-cutting biopsy guns (Fig. 12.2) have been used. Cutting-needle biopsy has been shown to be more sensitive than FNA biopsy in diagnosing pleural malignancies [14, 33]. The size of the needle (14 vs. 18 gauge) used in cutting-needle biopsy does not influence diagnostic sensitivity [14, 34]. The use of FNA biopsy and core biopsy together may help increase the diagnostic yield for malignant disease [14, 35].
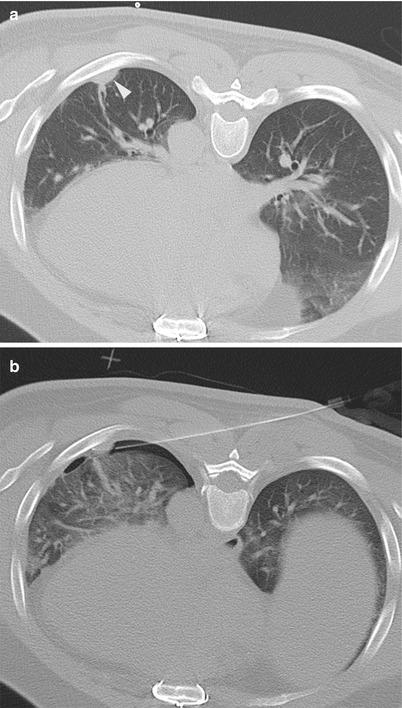

Fig. 12.7
A 46-year-old man with history of thymoma returns after definitive therapy with a focal pleural nodule. Axial CT image of the chest in prone position (a) shows a small focal area of pleural thickening covered by the left 7th rib. Initial attempts at accessing the nodule led to development of a small pneumothorax, but the biopsy was ultimately completed (b). Small pneumothorax remained stable and did not require any further intervention. Pathology confirmed recurrent thymoma
Approaches and Relevant Anatomy
In ultrasonography, patients can be positioned in a sitting or a recumbent position. A sitting position is advantageous in patients with large pleural effusions to avoid worsening of respiratory symptoms such as shortness of breath, which is frequently encountered in these patients. Positioning of the patient for CT-guided biopsy depends on the location of the target tissue. Patients can be placed in a supine, prone, lateral decubitus, or oblique position. Tilting the CT gantry helps target lesions located behind a rib. To biopsy areas of minimal pleural thickening, the needle should be aligned almost parallel to the pleural surface that is to be biopsied to allow the longest interface of the needle with the area of abnormality (Fig. 12.2). This is important for both FNA and tissue-cutting biopsies.
Potential Complications
Potential complications of pleural biopsies include pneumothorax, hemothorax, hemoptysis, subcutaneous hematoma, damage to the diaphragm or abdominal viscera, and tract seeding. For image-guided biopsies, the complication rate is less than 5 %. For blind pleural biopsies, the complication rate is higher, with 15 % of patients developing pneumothorax and 5 % of patients developing hemothorax or vasovagal reactions (Table 12.1) [14, 34–41].
Table 12.1
Image-guided pleural biopsy outcomes and complications
References | Patients/biopsies | Imaging | Needle | Outcome | Complications | ||
|---|---|---|---|---|---|---|---|
Sensitivity | Specificity | Accuracyor adequacy | |||||
Adamsand Gleeson [14] | 33/33 | CT 24 | Core needle 18and 14 G | 88 % | 100 % | 91 % | 1 chest wallhematoma |
US 9 | |||||||
Heilo et al. [34] | 70a/70 | US | Core needle 18and 14 G | 77 % | 88 % | 80 % | 1 hemoptysis |
1 chest pain | |||||||
Scott et al. [35] | 42/45 | CT | Core 18 G | 83 % | 100 % | 92 % | 1 hemoptysis |
1 stroke | |||||||
Agrawal et al. [36] | 22a/23 | CT 10
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||