Alveolar pneumonia: the inflammatory reaction is primarily confined to the airspaces and has three different manifestations:
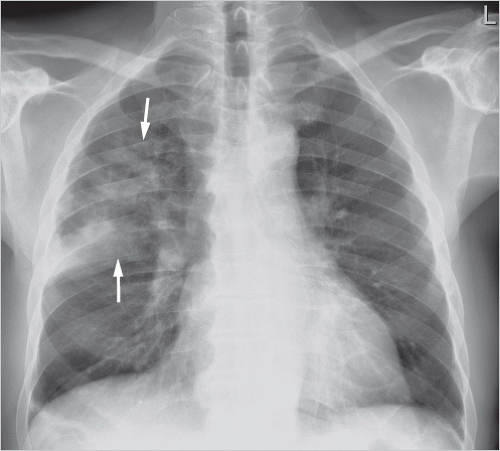
Lobar pneumonia: one entire lung lobe is evenly affected, attesting to microbial spread via the bronchial system and pores of Kohn. Lobar pneumonia follows a sequence of congestion → red hepatization → gray hepatization → yellow hepatization → resolution. Extensive consolidation of the affected lobe of lung is seen radiologically (▶Fig. 5.1).
Bronchopneumonia: bronchogenic microbial spread results in inflammation of the peribronchial lung parenchyma, where there is less extensive spread than that seen in lobar pneumonia. This is the most common type of pneumonia. Imaging shows peribronchial ground-glass opacity and consolidation, which again are less extensive compared with lobar pneumonia. These opacities are not found throughout the entire lobe; often, they are seen simultaneously in several lobes (▶Fig. 5.2).
Focal pneumonia: this manifests as a local focus of inflammation that does not spread further within the lung parenchyma. Often, focal pneumonia is asymptomatic or oligosymptomatic, making it difficult on differential diagnosis to distinguish this from lung carcinoma since both conditions manifest as consolidation or focal ground-glass opacity (▶Fig. 5.3).
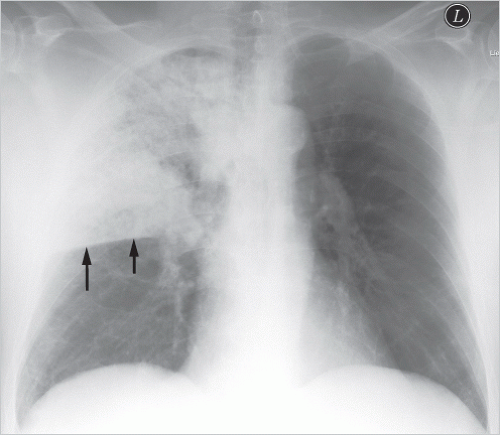
Fig. 5.1 Lobar pneumonia in the right upper lobe. Radiograph. The inferior margin of the consolidation is sharply bounded by the minor fissure (arrows); the middle lobe is not affected.
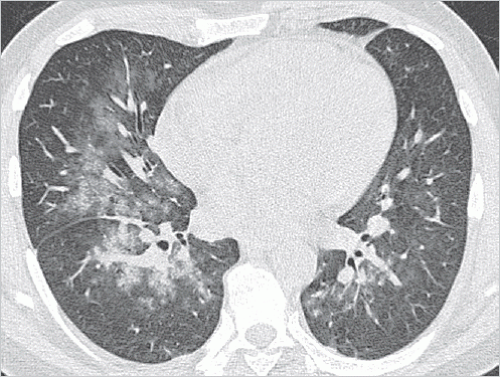
Fig. 5.2 Bronchopneumonia in the middle lobe and in both lower lobes. CT image.
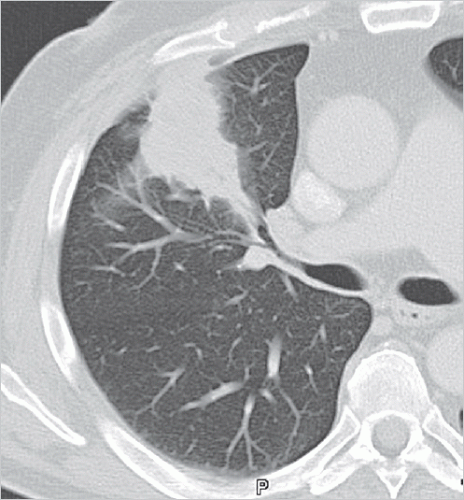
Fig. 5.3 Focal pneumonia in the right upper lobe. CT image.
Interstitial pneumonia: the inflammatory reaction unfolds primarily in the lung interstitium, as characteristically observed for intracellular pathogens (e.g., viruses, chlamydiae). The predominant radiologic finding is ground-glass opacity (▶Fig. 5.4).
Table 5.1 Characteristic findings in pneumonia and typical microbial spectrum11 | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pleural empyema: pleural empyema generally manifests as a rather large pleural effusion that is often multiloculated rather than free-flowing. It is not possible radiologically to make a reliable distinction between parapneumonic pleural empyema and noninfected, sympathetic pleural
effusion (see criteria below suggestive of pleural empyema). In cases of justified clinical suspicion (no improvement, deterioration of general condition, or persistently high inflammatory laboratory results), differentiation can be made between pleural empyema and noninfected pleural effusion through thoracocentesis. This procedure is recommended for hospitalized patients whose lateral upright chest radiograph shows a pleural effusion of more than 5 cm.1

Fig. 5.4 Interstitial pneumonia. CT image. Diffuse, bilateral ground-glass opacity.

Fig. 5.5 Pneumonia in the right upper lobe. CT image. Central cavitation in consolidations.

Fig. 5.6 Lung abscess in the left lower lobe. CT images. (a) Soft-tissue window: relatively thin-walled cavitation. (b) Lung window: air-fluid level in abscess.

Fig. 5.7 Focal pneumonia in the left upper lobe. CT image
Abscess formation: early abscess formation is seen on CT as rounded, largely water-isodense areas in lung regions affected by pneumonia. Later, following bronchial drainage of the liquid portions, abscess formation can be identified as cavitation on radiographs, too (see ▶Fig. 5.5). That antibiotics rarely penetrate into the inside of abscesses gives rise to a delayed treatment response and the need for a prolonged treatment course. CT examination is indicated if a lung abscess is suspected1 to rule out any mass, foreign body, infarction pneumonia, or superinfection caused by bronchial obstruction. If conservative antibiotic treatment fails, CT- or fluoroscopy-guided drainage may be useful.2

Fig. 5.8 Bilateral reticulonodular pattern in viral pneumonia. CT image.
Large, possibly multiloculated pleural effusion.
Delayed onset in relation to the underlying pneumonia.
Echogenic appearance on ultrasonography.
On CT, thickening and contrast enhancement of the parietal and visceral pleura (split pleura sign).
Persistently high or rising titers of inflammatory laboratory results despite regressing pneumonia.
 Fig. 5.9 Miliary pattern in miliary tuberculosis. CT image. |
Bilateral ground-glass opacity.
Bilateral, poorly marginated nodules.
Bilateral, in the later course confluent, consolidation.
Reticular opacity.
Small centrilobular nodules.
Hyperinflation of the lung parenchyma.
Pleural effusion.
Hilar lymphadenopathy (in children).
Respiratory rate: at least 30/min.
Diastolic blood pressure: maximum 60 mm Hg.
Systolic blood pressure: maximum 90 mm Hg.
Clouding of consciousness: present.
Age: at least 65 years.
Table 5.2 Characteristics of typical and atypical pneumonia | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 5.3 Risk stratification and diagnostic scope for community-acquired pneumonia1 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||
Treatment failure:
Progressive pneumonia:
Deterioration of clinical state (respiratory insufficiency, severe sepsis, or septic shock).
Radiologic progression seen only coincident with clinical deterioration.
Delayed-response pneumonia: no clinical stability observed within 72 h of starting treatment (stability criteria: heart rate, maximum 100/min; respiratory rate, maximum 24/min; systolic blood pressure, at least 90 mm Hg; body temperature, maximum 37.8°C; oxygen partial pressure, at least 60 mm Hg; and oxygen saturation, at least 90%).
Suspected lung abscess.
Progressive pneumonia: any increase in pulmonary opacities seen on radiographs within the first 72 h may be interpreted as progressive pneumonia only in the setting of coincident clinical deterioration.
Delayed-response pneumonia: if there is no evidence of clinical stability within 72 h, other causes, also nonmicrobial, for the pulmonary opacities must be considered.
resistance, together with new types emerging during treatment, the clinical and radiologic course is more challenging compared with community-acquired pneumonia. Multifocal infiltrates and changing courses alternating between regressive and progressive findings are common (▶Fig. 5.12).
Table 5.4 Differential diagnosis of hospital-acquired (nosocomial) pneumonia in intensive care patients with pulmonary opacity on radiograph8 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Aplasia: this is defined as a decline in the neutrophil count to below 500/µL or in the leukocyte count to below 1,000/µL, often secondary to aplasiogenic chemo- or radiotherapy. This leads to diminished phagocytosis. The lung infections presenting during the initial days of onset of aplasia are often imputed to gram-positive cocci, especially S. aureus, and gram-negative bacteria, such as Pseudomonas aeruginosa. If aplasia persists for at least 5 days, fungal infections are identified additionally. In Europe, these are typically caused by Aspergillus and Candida species.9
AIDS (acquired immunodeficiency syndrome): this leads to a reduction in the T helper cells (CD4-positive T lymphocytes) and, in turn, to reduced specific cellular immunity. The most common pulmonary infection is Pneumocystis jirovecii pneumonia, which is frequently the first manifestation of AIDS disease. Less commonly, tuberculosis and pulmonary toxoplasmosis are observed.9
Organ transplantations: organ transplant results in reduced lymphocyte function due to immunosuppression, putting patients at risk for pneumonia caused by intracellular pathogens, such as Legionella pneumophila, and viruses (especially cytomegalovirus), as well as for mycobacteriosis, nocardiosis, and aspergillosis. Aspergillus and Nocardia account for two-thirds of infections in heart transplant patients. Lung transplant recipients are at higher risk for cytomegalovirus infection.9
Stem cell transplant: in the conditioning phase, the patient’s diseased bone marrow is destroyed by means of high-dose
chemotherapy and whole-body irradiation, making the patient particularly susceptible to pneumococcal pneumonia during this phase. In the wake of conditioning and stem cell transplant, the risk of opportunistic pneumonia is similar to that faced by aplastic patients.
Immune mechanism
Mediators
Damage
Spectrum of opportunistic microorganisms
Adaptive cellular response
T cell system
Organ transplant
Stem cell transplant
Cortisone treatment
Hodgkin lymphoma
Chronic lymphatic leukemia
Pneumocystis jirovecii
Viruses (cytomegalovirus, herpes simplex virus)
Mycobacteria
Legionellae
Adaptive humoral response
Immunoglobulins
Chemotherapy
Malignant melanoma
Chronic lymphatic leukemia
Hypogammaglobulinemia
Pneumococci
Gram-negative bacteria
Mycobacteria
Candida
Pneumocystis jirovecii
Viruses (cytomegalovirus, herpes simplex virus)
Innate cellular response
Macrophages/monocytes
Granulocytes
NK cells
Chemotherapy
Radiotherapy
Stem cell transplant
Blood system disease
Bone marrow carcinosis (e.g., small cell lung carcinoma)
Staphylococcus aureus
Gram-negative bacteria
Fungi
Innate humoral response
Complement system
Properdin system
Monokines/lymphokines
Multiple myeloma
Non-Hodgkin lymphoma
Chronic lymphatic leukemia
Streptococcus pneumoniae
Haemophilus influenzae
Abbreviations: AIDS, acquired immunodeficiency syndrome; NK cells, natural killer cells.
High-dose corticosteroid treatment: this results in impairment of granulocyte function, thus compromising chemotactic activity. Patients are at increased risk for P. jirovecii pneumonia. Preexisting tuberculosis may be reactivated.9
with the force of gravity and this can serve as a diagnostic criterion. At times, the aspergilloma may fill the entire cavern and can no longer be distinguished from the cavern wall; instead, the entire lesion appears as a relatively homogeneous area of consolidation (▶Fig. 5.14). Noninvasive aspergilloma can progress to an invasive form of Aspergillus infection in immunocompromised patients.
 Fig. 5.14 Aspergilloma in the right upper lobe. CT image. The fungus ball cannot be distinguished from the cavern wall; the entire lesion manifests as a homogeneous nodule. |
 Fig. 5.15 Invasive pulmonary aspergillosis in the right upper lobe. CT images. Course over 2 weeks. (a) Baseline findings in aplastic patient: rounded consolidation in the right upper lobe, surrounded by ground-glass opacity, i.e., halo sign. (b) Two weeks later, patient is no longer aplastic: newly emerged air crescent sign (arrows), slight increase in nodule size.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|