|
Define what point-of-care sonography (POCUS) encompasses and how it is being utilized.
List some of the primary applications for POCUS.
Explain the common acoustic windows, probe placement, and anatomy seen in certain types of examinations.
Discuss the importance of evaluating certain anatomical perspectives for possible pathologies.
Demonstrate the clinical application and sonographic differentiation for certain types of limited examinations.
mechanical compression of the heart resulting from large amounts of fluid collecting in the pericardial space and limiting the heart’s normal range of motion
refers to normal vein walls touching with compression
the formation or presence of a thrombus within a vein
a surgical procedure used to insert a catheter through the abdominal wall and fascia; a syringe is attached to the catheter to aspirate fluid; bleeding is confirmed when gross blood is aspirated; saline solution is injected into the catheter, then it is drained and analyzed
accumulation of blood in the pleural cavity (the space between the lungs and the walls of the chest)
blunt force injury specific to the gastrointestinal tract system occasionally resulting in perforation and leakage of enteric contents
incision made into the abdomen to insert a camera (laparoscope) into the abdomen to visualize and examine the abdomen and pelvic structures and spaces
inside space of a tubular or cellular structure
pleura that lines the inner chest walls and covers the diaphragm
presence of fluid within the pericardium
the abnormal presence of air between the lung and the wall of the chest (pleural cavity), resulting in collapse of the lung
clinician who has the ability to both perform and interpret bedside ultrasonography
presence of abnormal quantities of air within subcutaneous tissue
pleura that covers the lungs
TABLE 25-1 Medical Specialties Currently Using Point-of-Care Ultrasonography Applications11 | |||
|---|---|---|---|
|
outpatient settings.11,12 As with any growth, there are continued challenges to ensure whether its full potential and diagnostic abilities are being reached. These include but are not limited to adequate training, proper documentation and image archiving for all to adhere to, meeting standard competencies, and proper governance or oversight. Once these important criteria have been established and fulfilled, proper continuing education requirements should be met, and the necessary skill sets should continually be developed. Another important factor to successful POCUS adaptation is the utilization of proper equipment. The lack of proper equipment may limit the type of applications available for patient care and the thoroughness of its adoption.
|
TABLE 25-3 Outline of the 12 Emergency Medicine Core Applications | ||
|---|---|---|
|
noninvasive bedside examination, which can be performed on pregnant women and children, without the risk of exposure to ionizing radiation or nephrotoxic contrast agents.
Is there free fluid in the abdomen?
Is there free fluid in the thorax?
Is there increased fluid in the pericardium?
Is there evidence of a pneumothorax?
Is this causing patient symptoms?
1. Right upper quadrant view (RUQ)
2. Left upper quadrant view (LUQ)
3. Pelvic/bladder view
4. Cardiac view (parasternal long axis or subxiphoid)
5. Lungs (right and left)
pouch in males or rectouterine pouch in females (Fig. 25-4A). A Trendelenburg position may be used if satisfactory images are not obtained with the patient in a supine position. Evaluation of the pelvic cavity using both longitudinal and transverse planes should be performed.
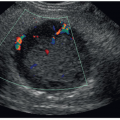
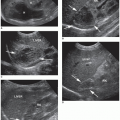
 FIGURE 25-3 Coronal image of the left upper quadrant. The sonogram demonstrates the relationship of the spleen (S) and the left kidney (K). There is no anechoic blood or fluid identified. |
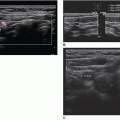
and convenient method to sonographically visualize cardiac structures, including the pericardial sac. With the transducer oriented transversely and angled superiorly in the subxiphoid region/window, the four-chamber cardiac image can be recognized. The liver serves as an acoustic window, and often, a small segment of the liver can be visualized in the near field. The base of the heart, including both atria, should be located to the patient’s right and is slightly posterior. The apex of the heart is located more to the patient’s left and is situated more anteriorly and inferiorly. If any of the four chambers are not fully visualized within this acoustic window, an attempt should be made to adjust the transducer orientation, so that it is almost parallel to the skin of the anterior torso. In certain patients, especially those with abdominal distention or pain, the subxiphoid window may not be optimal; therefore, familiarity and mastery of all cardiac scanning planes, discussed below, will help to rule out any pericardial or cardiac pathology.29
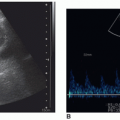
once the echogenic reflectors (parietal pleura and visceral pleura) just distal to the ribs are seen in real time with the visceral pleura sliding back and forth under the parietal pleura with patient respiration (Fig. 25-6B). The sliding sign and moving comet tail artifacts are synchronized with respiratory movement. Absence of the sliding sign indicates that there is a possible pneumothorax. Finding a sliding sign can immediately rule out a pneumothorax at this particular location; however, multiple areas should be evaluated, especially in the absence of the sliding sign. Although this imaging finding can be present along any and all acoustic windows in the upper thorax, with the patient in the supine position, the more superior location is the common site for a pneumothorax.
and normal lung sliding can be demonstrated between the pneumothorax and the normal lung.27 At the lung point position during expiration, no sliding is seen; but, with inspiration, the lung inflates and the visceral pleura moves up in apposition with the parietal pleura beneath the probe and sliding is again seen.35 The ability to demonstrate the alternating lung sliding and absence of lung sliding within the same field is diagnostic of pneumothorax with a sensitivity of 66% and specificity of 100%.26, 27 and 28
Is there evidence of gallstones?
Is there biliary wall thickening?
Is there fluid surrounding the biliary wall/pericholecystic fluid?
Is there a sonographic Murphy sign?
head to widen the intercostal spaces. If the ribs are still blocking the view, the patient can be asked to hold a deep breath to further widen the intercostal spaces. Additionally, the probe can be rotated slightly obliquely to align with the intercostal spaces.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree