Ajita Deodhar • John A. Kaufman
Polyvinyl alcohol (PVA) is one of the oldest, particulate embolic materials providing inexpensive, permanent occlusion of blood vessels. It is a water-soluble, colorless synthetic polymer made from polyvinyl acetate through partial or full hydrolysis to remove the acetate groups. The extent of hydroxylation determines the physical, chemical, and mechanical properties of the PVA.1 Typically, PVA is highly soluble in water but resistant to most organic solvents, which allows it to be used for many applications, including paper manufacturing, cosmetics, household sponges, food packaging, and medical devices.2 The first ever medical use of PVA was reported by Grindlay3 in 1949 at the Mayo Clinic as a prosthesis after pneumonectomy. Since then, it has found multiple medical applications such as cardiac surgery, skin grafting, embolic material, artificial cartilage, artificial tear replacement, etc.2 Its nontoxic, inert properties have been well established over the last several decades. Tadavarthy et al.4,5 was the first to report the use of PVA as an embolic material in the mid-1970s. It was used to treat patients with cervical carcinoma, hemangiosarcoma of the liver, hemangioendothelioma of the neck and forehead, and an arteriovenous malformation of the spine.
DEVICE/MATERIAL DESCRIPTION
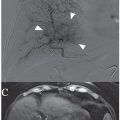
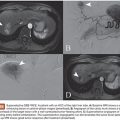
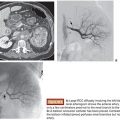
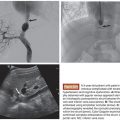
PVA is most commonly available as an intrinsically nonvisible occlusive agent that is typically used in combination with contrast to be radiographically visible. The preparation of PVA particles first involves its conversion into a foam that can absorb water and become readily compressible. Historically, sheets or blocks of dried foam were shaved to yield irregular particles of varying sizes. The resulting shavings or particles were then passed through sieves with sequentially smaller holes to separate them into various sizes.6 Given the irregular configuration of each individual particle, it was possible for larger particles to pass through small holes depending on its orientation as it passes through the sieve. This explains why there was variability in early particle preparations.7 Today, PVA is supplied as a preparation of irregular or spherical particles within a standardized size range (Fig. 6.1), although the potential for size variability still exists within the nonspherical preparations. This is a potential issue when using particulate PVA because the presence of smaller particles than anticipated may lead to uncontrolled distal embolization (with tissue infarction), whereas the presence of larger particles than anticipated may lead to proximal embolization (with potential recanalization).

PVA is extremely resilient and compressible with excellent memory, allowing it to regain its shape and size once it comes in contact with body fluids.7 In fact, due to its inherent memory, PVA particles have the potential to expand approximately 4 to 15 times once they come in contact with solution and can therefore occlude blood vessels slightly larger than the internal diameter of the catheter.7 In addition, the particles have a tendency to clump together when suspended in saline. Therefore, the effective size of this agent is often larger than that of the individual dried particles, which can contribute to a more proximal occlusion than intended.8 This property can also increase the risk of microcatheter occlusion during delivery.
MECHANISM OF ACTION
The administration of PVA particles initially leads to slow flow due to adherence of the particles to the vessel wall.9 This ultimately leads to an inflammatory reaction, a foreign body reaction, and thrombosis.10–12 PVA is a nonbiodegradable embolic agent that has traditionally been thought to lead to a permanent vascular occlusion.7 This occurs with organization of thrombus, disappearance of the inflammatory infiltrate, and ingrowth of connective tissue resulting in fibrosis. However, luminal recanalization after embolization with PVA has been reported as well, which may be due to resorption of thrombus and/or angiogenesis and capillary regrowth caused by vascular proliferation inside the organized thrombus.9,12,13
TECHNIQUE
Before using PVA as an embolic material, particulate PVA should be reconstituted to allow for radiographic visualization during delivery. This can be achieved by adding contrast, barium sulfate 60% or tantalum powder. To decrease particle clumping, albumin, dextran, absolute alcohol, or absorbable gelatin foam may be added to the saline suspension.2
PVA embolization uses a flow-directed technique and is performed under fluoroscopic guidance. During embolization, it is therefore necessary to monitor the administration at all times to quickly recognize when antegrade flow is slowing and vascular occlusion has taken place. Failure to recognize the slowing and changing direction of flow can increase the possibility of nontarget embolization due to particle reflux out of the target vessel. Given the tendencies of these particles to clump, arterial occlusion may occur faster than anticipated. In addition, frequent catheter flushing is recommended to minimize the possibility of catheter occlusion.
CLINICAL APPLICATIONS
PVA finds application wherever particulate embolization of a permanent nature is required. In general, this includes gastrointestinal or internal hemorrhage secondary to trauma, anticoagulation, etc.; therapeutic or presurgical tumor embolization; and embolization of uterine fibroids (uterine artery embolization).
POTENTIAL COMPLICATIONS
The complications reported in association with embolization using PVA particles have typically been related to the organ and pathology being embolized as opposed to the embolic agent itself. However, complications related to the characteristics of PVA can occur and are typically a function of flow. As described, the characteristics of PVA particles can lead to particle clumping, leading to proximal embolization with a potential for subsequent recanalization and procedural failure. Avoiding particle clumping and nontarget embolization requires attention to detail while preparing and delivering PVA.
TIPS AND TRICKS
• PVA particles should be matched to the size of the arteries to be occluded.
• The particles should be delivered in small aliquots with a 1-mL Luer lock syringe using road map imaging to monitor for flow and reflux.
• After reaching the desired end point, it is prudent to wait for 5 min and then perform another angiogram to check for return of flow due to distal migration of clumped PVA.
• Adding a little 25% albumin to the contrast/saline dilutant (1:20) minimizes clumping in the syringe. If the delivery catheter is blocked with particles, it can be cleared with a 1-mL Luer lock saline syringe.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree