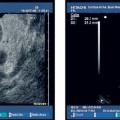
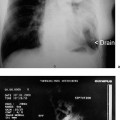
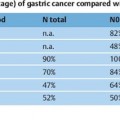
34 Portal Hypertension and Endoscopic Ultrasound Portal hypertension is a pathologic increase in portal venous pressure. It results in a clinical syndrome that may include the development of varices, ascites, and encephalopathy. Although intrinsic liver disease manifesting as cirrhosis is the most common cause in Western countries, presinusoidal portal hypertension due to schistosomiasis is the predominant cause worldwide.1 Gastroesophageal varices have two main inflow tracts, the first being the left gastric or coronary vein, which decompress the portal vein directly (when flow is hepatofugal). The other major route is via the short gastric veins, which decompress the splenic vein at the splenic hilum and are often clinically relevant when splenic vein thrombosis results in isolated fundic varices in the stomach. One of the most detailed examinations of the venous anatomy of the lower esophagus and upper stomach was reported by Hashizume et al. in 14 cadaver specimens (with portal hypertension in nine cases).2 The authors demonstrated that, superficially, there are intraepithelial channels that collect and join to form a venous plexus just below the epithelium. The superficial venous plexus and adjacent deep submucosal veins are contiguous from the lower esophagus to the upper stomach and combine to form the endoscopically visible submucosal varices above and below the esophagogastric junction. Finally, adventitial para-esophageal collateral veins connect to the submucosal veins via perforating veins that penetrate through the muscularis propria. These para-esophageal collateral veins have since been classified, using a 20-MHz ultrasound probe, as peri-esophageal collateral veins (peri-ECVs) if they are directly in contact with the muscularis propria, and as para-esophageal collateral veins (para-ECVs) if they are not.3 An analogous classification for gastric collateral veins (GCVs) into peri-GCVs and para-GCVs has been suggested. In addition, the study by Hashizume et al. showed that the anterior branch of the left gastric vein forms gastroesophageal varices by directly communicating with submucosal veins (the anterior branch), while the posterior branch of the left gastric vein extends along the outside layer of the esophageal wall across the esophagogastric junction to the aforementioned para-esophageal veins.2 In summary, gastroesophageal varices form as a result of a complex but well-described venous circulation. In early studies, endoscopic ultrasonography (EUS) was found to be inferior to upper esophagogastroduodenoscopy (EGD) for detecting esophageal varices.4–6 This was thought to be due to mechanical compression of esophageal varices by the endoscope and the EUS balloon, as well as the fact that the echoendoscope had a focal length outside the range of the varices.7 Initially, the maximum reported sensitivity of EUS for esophageal varices was less than 60 % relative to EGD. It must be borne in mind that endoscopic grading of varices has been shown to be subject to marked interobserver variability, including variability regarding the presence or absence of red signs.8 However, when a 20-MHz miniprobe was used, the results were better. Using miniprobes, Miller et al. demonstrated that the mean variceal circumference increased with increasing endoscopic variceal grade, although overall correlation was poor.8 In contrast, Faigel et al. found that, in comparison with EGD, EUS was similar for detecting esophageal varices (72% versus 74%, respectively).9 In 2002, Lee et al. compared cirrhotic and dyspeptic (control) individuals.10 There was good agreement between using EUS and EGD for the endoscopic (luminal) diagnosis of esophageal varices. Using EGD as the gold standard, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of EUS for esophageal varices were each 96 %. EUS appears to be clearly superior to endoscopy for detecting gastric varices.4–6,9 Using EUS as the gold standard, the sensitivity, specificity, PPV, and NPV of esophago-gastroduodenoscopy for gastric varices were 44%, 94%, 78%, and 79%, respectively.10 Another study reported a sensitivity of 70% with EGD, but the group of patients included had larger varices overall.11 In summary, EUS appears to provide similar results to EGD for detecting esophageal varices and is superior to EGD for detecting gastric varices. The use of higher-frequency miniprobes may improve the performance of EUS with regard to esophageal varices. Before the advent of EUS, imaging of the collateral venous system required venography, which is invasive, and/or computed tomography (CT), which has inadequate sensitivity.10 Although one study demonstrated that multidetector-row CT angiography and EUS were equivalent for fundic gastric collateral varices,12 CT is still inferior for examining wall thickness and blood flow velocity.13 With regard to the esophageal vasculature, Lee et al. detected both peri-ECVs and para-ECVs in two-thirds of cirrhotic patients.10 The size of the varices correlated positively with the Child-Pugh score and esophageal varix size.9,10 In addition, 93 % of patients with varices had perforating veins connecting to ECVs, and the remaining two patients had esophageal varices connected to GCVs. All perforators were found within 5 cm above the gastroesophageal junction. However, the sensitivity for perforating veins ranges from 15–100 % in the literature, depending on the technique used and the size of varices.10,14–16 With regard to gastric collaterals, Lee et al.10 found para-GCVs in 81 % of cirrhotic patients and peri-GCVs in 65 %. The number of perforating veins correlated positively with the Child-Pugh score and the sizes of both esophageal varices and gastric varices. Faigel et al. showed that large para-GCVs (>5mm) correlated with large para-ECVs (>5mm).9 Using EUS alone in cirrhotic patients, a diagnosis of portal hypertension could be made on the basis of venous abnormalities with a sensitivity, specificity, PPV, and NPV of 92%, 95%, 84%, and 98%, respectively.10 By contrast, diagnosis of portal hypertension with EGD in cirrhotic patients had a sensitivity, specificity, PPV, and NPV of 58%, 100%, 100%, and 89%, respectively. Another study similarly found that EUS detection of ECVs was 97% sensitive and 97 % specific for the presence of cirrhosis,9 while the EGD diagnosis of esophageal varices only had a sensitivity of74% for cirrhosis. In patients without portal hypertension, venous abnormalities were only detectable in 5%of cases in one study,10 but 22 % were found to have perigastric collaterals in another.9 The latter authors argued that small gastric collateral varices may be a normal finding at EUS, but that ECVs signify portal hypertension. Kakutani et al. identified gastrorenal shunts in patients with gastric varices and reported a95% rate of agreement (K = 0.9) between contrast-enhanced CT and EUS.17 Two additional shunts found only on EUS were < 5 mm in size. The presence of these shunts may allow balloon-occluded retrograde transvenous obliteration. Bastid et al. subsequently commented that transabdominal Doppler ultrasound should continue to be the first-line examination in assessing these patients.18 In summary, EUS can easily visualize ECVs, GCVs, and often perforating veins. The size of the collaterals and the number of perforating veins is associated with the size of the gastroesophageal varices. EUS visualization of varices and/or collateral circulation correlates well with the presence of portal hypertension. EUS has made it possible to visualize ectopic varices in a variety of locations. Yeh et al. published a case report on duodenal varices.19 Palazzo et al. reviewed cases of extrahepatic biliary obstruction resulting from biliary varices.20 Except for a patient who underwent portal venography, each patient had undergone transabdominal ultrasonography and abdominal CT that did not detect the varices. All patients had portal vein thrombosis, which was seen as a solid, echogenic thrombus on EUS. Biliary varices were found both in and around the wall of the common bile duct, as well as in the gallbladder wall. Another case of portal vein thrombosis with choledocholithiasis was reported with biliary varices.21 Rai et al. suggested that intraductal ultrasound may better visualize biliary varices causing obstructive jaundice.22 EUS also diagnosed choledochojejunal anastomotic varices following total pancreatectomy.23 EUS detection of varices in the lower gastrointestinal tract includes pancolonic varices in a patient with idiopathic portal hypertension,24 but most commonly involves rectal varices. Dhiman et al. demonstrated submucosal rectal varices that were not visible on endoscopy.25 More severe rectal varices occurred in patients with large esophageal varices and a history of injection sclerotherapy. There was no correlation between the severity of hemorrhoids and portal hypertension. In another study using endoscopic color Doppler ultrasound (see next section), color flow imaging identified the varices and their afferent vessels in 12 patients with rectal varices.26 All had a continuous flow wave, except one with a pulsatile component. In summary, EUS can help identify ectopic varices if the site is endoscopically accessible. ECDUS allows hemodynamic examination of blood vessels and displays the direction of flow. It has been shown to enhance EUS imaging of gastroesophageal varices. Sato et al. have published extensively on the use of ECDUS and gastroesophageal varix detection. Color flow images of esophageal varices and esophageal collateral veins, as well as of gastric varices and gastric collateral varices, were obtained in all patients with gastroesophageal varices.13,27 The wall thickness of gastric varices with red color signs, erosions, or associated bleeding was significantly less than with other gastric varices. The mean flow velocity in gastric varices was also significantly higher in larger varices and in cases with associated bleeding. The authors therefore suggested that flow velocity and wall thickness measurement may be useful for predicting bleeding in patients with gastric varices.13 However, this has not been confirmed in prospective studies. As stated, the detection rate for perforating veins in the literature has varied widely, from 15% to 100 %.10,14–16 Perforating veins have mostly afferent flow (i. e., toward the submucosal varix), as shown by ECDUS,3,15,28 and are associated with larger varices.14,28 Using the galactose-based Doppler-enhancing contrast agent Levovist (Schering, Berlin, Germany), the yield for diagnosing perforating veins improved from 77 %to97% and the direction of flow was subjectively assessed more easily.16 The same group used Levovist to detect recurrent varices, and the sensitivity of ECDUS detection of perforating veins rose from 31 % to 76 %.15 Levovist also helped improve ECDUS detection of arterial blood flow in esophageal varices.29 The authors suggest that only afferent perforators (type I) should be targeted with sclerotherapy, and they recommend avoiding efferent (type II) and mixed (type III) perforating veins, which may be serving to partially decompress the gastroesophageal varix. Wong et al. demonstrated a reproducible continuous venous hum in five patients with gastric varices and in one with pulsatile flow, allowing differentiation from non-variceal submucosal masses.30 Gastric varices due to splenic vein thrombosis were described using ECDUS color flow as having “round cardiac and fundal regions at the center,” with variceal extension to the greater curvature of the gastric body.31 Hino et al. used ECDUS to investigate the left gastric vein and its relation to the formation of gastroesophageal varices.28 Increased left gastric vein trunk diameter only showed a trend toward association with larger varices, but hepatofugal left gastric vein flow velocity did statistically increase with increasing variceal size. Transabdominal ultrasound has previously shown that flow velocity in the left gastric vein is associated with variceal bleeding.32 The dominance pattern of the left gastric vein was classified according to the relative sizes of its anterior and posterior branches into an “AD type” for anterior-branch dominance, a “PD type” for posterior branch dominance, and an “ND type” for nondominance (equal branch diameters). The AD type was associated significantly more often with larger variceal size. This was not surprising, as the anterior branch traveled to the esophagogastric junction and ultimately entered varices, whereas the posterior branch traveled via para-ECVs to enter the azygos system, and/or formed a renal shunt. However, the posterior branch may still affect variceal size and bleeding to some extent via perforating veins originating from the para-ECVs. Finally, Pontes et al. used an ECDUS balloon to carry out manometry of esophageal varices.33 The pressure when the Doppler signal disappeared was recorded. Good pressure correlation was observed in an in vitro model. It was possible to measure the esophageal varix pressure in 93 % of cases. This pressure and the portal pressure (estimated using the hepatic vein pressure gradient) correlated significantly (r = 0.64). In summary, hemodynamic assessment with ECDUS has improved our understanding of the pathophysiology of gastroesophageal varices. It appears that the left gastric vein has the greatest impact onvariceal formation—mainly through its anterior branch flowing into the area of the esophagogastric junction, but perhaps also via its posterior branch, forming collateral vessels that communicate with gastroesophageal varices, often via afferent-flowing perforating veins. Nonvariceal Markers of Portal Hypertension and Chronic Liver Disease It is well known that patients with cirrhosis may have ultrasonographic parenchymal abnormalities such as inhomogeneity, lobularity, irregularity of the liver surface, and prominence of the caudate lobe. Patients with active chronic hepatitis also often have large, benign-appearing perihepatic lymph nodes. Other indirect signs of portal hypertension include non-variceal vascular abnormalities and ascites. Although this subject has not been studied specifically in portal hypertension, EUS is likely able to detect very small pockets of ascites and early evidence of variceal congestion that cannot be detected with CT or transcutaneous ultrasound. Small-volume ascites detection has been shown to be feasible in the context of esophageal and gastric cancer.34,35 These results should apply to ascites in cirrhotic patients as well.
 Gastroesophageal Varices and Related Vasculature
Gastroesophageal Varices and Related Vasculature
 Visualization of Esophageal Varices and Gastric Varices
Visualization of Esophageal Varices and Gastric Varices
 Perforating and Collateral Veins
Perforating and Collateral Veins
 Hemodynamics using Endoscopic Color Doppler Ultrasound (ECDUS)
Hemodynamics using Endoscopic Color Doppler Ultrasound (ECDUS)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Ectopic Varices
Ectopic Varices