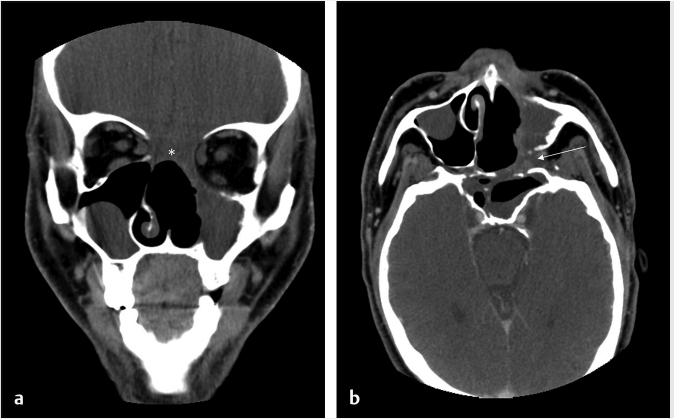
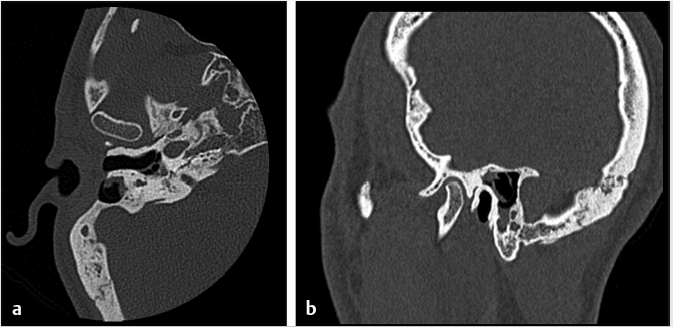
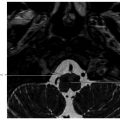
6 Posttreatment Appearance Following Skull Base Therapy Skull base tumors present a treatment challenge due the large number of critical structures that are present in an anatomically dense space. These tumors also require an equally sophisticated approach to intervention and imaging assessment. Single-modality treatment with surgery or radiation is the mainstay for the treatment of early-stage head and neck cancer.1 In the case of locally advanced disease, a multimodality treatment approach is employed, which combines surgery with adjuvant chemoradiation therapy. In the treatment of skull base cancers, both open craniofacial and endonasal approaches can be used independently or in concert. The postoperative imaging in these cases is notoriously challenging due to the alteration of the normal anatomy, the surgical defects, and any reconstructions. Moreover, a variety of tissue grafts and synthetic materials may be used in these reconstructions. An appreciation of the normally anticipated imaging features after skull base reconstruction lays the cornerstone for recognizing when there is a successful reconstruction as well as when there is the presence of residual disease or the development of a recurrence. Current follow-up guidelines from the National Comprehensive Cancer Network allow for a considerable degree of clinician discretion in surveillance practices. It is recommended that imaging and a physical examination be performed every 3 to 4 months for the first 2 years and every 4 to 6 months in years 2 to 5. Following this, follow-up is usually performed annually.2 This surveillance should not only focus on local recurrent disease, but also be vigilant to identify distant metastases and the development of second primary tumors. A wide array of imaging modalities is available to contribute to the assessment of the postoperative skull base. These include radiography/fluoroscopy, endoscopy, ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), single photon emission CT (SPECT), and 2-(fluorine 18) fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET), with or without CT (PET/CT) or MR (PET/MR). In many centers, CT is often used as the modality of choice for follow-up examinations as CT scanning allows for very good soft-tissue resolution and excellent assessment of bone and readily identifies cervical adenopathy. Rapidity of image acquisition is also a major advantage of CT, which makes it a great modality for scanning patients who are medically unstable or who cannot maintain a still supine position for prolonged periods. It is an ideal test to use in the early perioperative setting to diagnose acute complications. MRI has the advantage of even greater soft-tissue resolution and given that patients with skull base tumors may be at risk for dural invasion or perineural spread, contrast-enhanced MR is the modality of choice. MRI may also help in identifying suspected tumor recurrence. Diffusion-weighted imaging (DWI) MRI may be of particular utility in the early posttreatment period, where other modalities offer false-positive findings.1 DWI has also been reported to be a useful tool to differentiate tumor recurrence from normal posttreatment changes in the early period after treatment when used in combination with CT and/or MRI.3 Many centers also routinely utilize FDG PET/CT as the modality of choice in patient follow-up examinations. However, FDG PET/CT performed within the immediate 12 weeks after treatment may give both false-positive and false-negative results. The false-positive cases are primarily the result of treatmentrelated inflammation. The false-negative cases are the result of radiation and chemotherapy vascular compromise to the tumor bed and the suppression of glucose transporters in the tumor.1 Distinguishing postsurgical change from tumor recurrence is arguably the most important role that imaging serves following surgery. The challenge in assessment of these images lies in the often-extensive changes to normal anatomy that result from reconstruction, radiation therapy, and scarring. Clinically, recurrence may be asymptomatic and not come to attention until its bulk has caused gross distortion or is distinctly palpable. Imaging is therefore essential in the posttreatment surveillance of head and neck cancer. Skull base surgery requires intricate reconstructions that are frequently necessary to both maintain negative histopathological margins from a wide local excision and afford the patient minimal morbidity while closing the surgical defect. In this anatomically complex area, various flap reconstructions are used. These include local flaps, pedicle flaps, and free microvascular flap techniques.4,5 The flaps are intuitively named based on the tissue and vascular supply used. In the local flap technique, neighboring tissue is repositioned geometrically to accommodate the surgical defect. The pedicle flap technique differs in that donor tissue is rotated to fit the defect. The term pedicle denotes the use of the patient’s own tissue on its vascular pedicle to close the surgical defect. Finally, free flap techniques may employ either a simple free flap or composite free flap. Either variant requires microvascular anastomosis of the vascular pedicle of the free flap to the local recipient vascular supply in the region of the reconstruction. In the simple variant, only one type of tissue is incorporated into the flap, whereas the term composite belies the use of multiple tissue types. The most common forms of the latter are myocutaneous, fasciocutaneous, free jejunal interposition, and osseous composite flaps.6 Craniofacial resection (CFR) of the anterior cranial fossa is a radical surgery performed with the intention of complete tumor extirpation. This procedure is particularly common in the setting of adenocarcinoma (ADC), adenoid cystic carcinoma, chordoma, malignant melanoma, olfactory neuroblastoma, osteosarcoma, and squamous cell carcinoma. Prior to endoscopic surgery, CFR was the sole “gold-standard” procedure for tumors that involved the orbital roof, cribriform plate, and fovea ethmoidalis. Fig. 6.1 Coronal (a) and axial (b) computed tomography images following craniofacial resection of an inverted papilloma. The bony floor of the anterior cranial fossa, the nasal septum, and most of the turbinates have been resected. The reconstructed roof of the nasal cavity appears as a soft-tissue (asterisk; a) density beneath the frontal lobes. Note the smooth margins along the inferior margin of the flap. Axial image (b) also shows soft-tissue density in the left pterygopalatine fossa (arrow), which is compatible with scarring that can arise related to the left medial maxillectomy. The procedure typically begins with a lateral rhinotomy and is frequently followed by a medial maxillectomy, anterior and posterior ethmoidectomy, and sphenoidectomy. A lateral rhinotomy is commonly performed when tumor involves the deep nasal vault, the contiguous anterior paranasal sinuses, and midfacial skeleton. At surgery, the cribriform plate and frontal recesses are exposed along with the lamina papyracea bilaterally. A bicoronal forehead flap and frontal craniotomy are then performed to allow access to disease if it has extended into the anterior cranial fossa. Structures invaded by tumor are removed. The procedure may also require orbital exenteration. Postoperative imaging will show a craniotomy involving the frontal bone from the level of the glabella inferiorly and to roughly 4 cm above the skull base superiorly. Typically the midpupillary line serves as the lateral craniotomy border.7 Followup imaging will demonstrate resection of the cribriform plate, fovea ethmoidalis, and planum sphenoidale. Partial or near-complete absence of the nasal septum and turbinates may be evident as well as cranialization of the frontal sinuses ( Some surgeons interpose fat graft into the reconstruction site. This will manifest as a foci of fat attenuation on CT or as foci of T1 hyperintensity on MRI ( As anterior skull base tumors may invade or originate from the paranasal sinuses, one specific challenge on follow-up imaging is to distinguish between recurrent or residual tumor and retained secretions. Residual or recurrent tumors manifest as an intermediate signal on T1- and T2-weighted images and demonstrate mild internal enhancement. Secretions may show peripheral enhancement and have low T1 and bright T2 signal. More desiccated or proteinaceous secretions may often demonstrate very bright T1 and/or T2 signal intensities. Findings suspicious for recurrence would include an enhancing rounded mass along the resection margins and the development of new lesions with or without associated edema ( Lateral approaches to the skull base are most commonly employed in the setting of vestibular schwannomas, paragangliomas, recurrent tumors of the nasopharynx, and primary tumors of the external auditory canal/middle ear. The approaches are varied. Most involve the removal of bone and retraction of brain parenchyma for tumor removal. They can also involve rerouting of the facial nerve and identification of the internal carotid artery. In labyrinthine preservation procedures, the otic capsule is left intact and the labyrinth bypassed. Imaging will commonly show distortion of the normal anatomy along the surgical planes of dissection, which is usually transpetrous or transtemporal. Fig. 6.3 Gadolinium-enhanced T1-weighted imaging with fat saturation post craniofacial resection for a frontal sinus osteosarcoma. (a) A study done as a baseline following surgery. Note the avid enhancement in the reconstructed floor of the cranial fossa (asterisk) and the diffuse dural thickening and enhancement. (b) An examination done a few years later. Note that the flap reconstruction now shows much less enhancement and the resolution of dural thickening and enhancement. The cranial and inferior margins of the flap also show very smooth margins. Fig. 6.4 Coronal T2-weighted (a) and sagittal T1-weighted (b) images following resection of a sinonasal undifferentiated carcinoma. Note the bright signal in the bifrontal white matter in keeping with encephalomalacia. The reconstructed floor is also more heterogeneous and lobulated than in the other example ( Fig. 6.6 Coronal computed tomography (a) and sagittal T1-weighted (b) surveillance image studies showing expected changes following resection of an inverted papilloma with foci of squamous cell carcinoma. Subsequent coronal (c) and sagittal (d) magnetic resonance imaging shows recurrence in the nasal cavity along the margin of the reconstructed anterior cranial fossa floor (arrows). CT is essential in the postoperative period not only for the assessment of a postoperative complication, but also to document the bony anatomy and expected surgical change. Following this, MRI alone is often adequate for further follow-up, although some advocate the use of both modalities especially in the follow-up of petrous cholesteatoma.9 It is important to note that many variations on these procedures can be performed and that postsurgical findings can vary in terms of the approach and structures removed. A simple (or cortical) mastoidectomy involves removing the lateral wall of the mastoid bone and cortical mastoid air cells with preservation of the external auditory canal. No manipulation of the ossicular chain occurs with this technique. There is no removal of bone in the epitympanum. By comparison, a canal wall-up mastoidectomy (or combined approach technique) involves removal of the mastoid air cells and Koerner’s septum ( A canal wall-down mastoidectomy begins with a canal-up procedure and is then followed by removal of the external auditory canal. This allows for formal communication between the external auditory canal and the mastoid cavity ( Transpetrous approach techniques may be employed for many skull base tumors but are frequently performed for the removal of vestibular schwannomas, meningiomas, arachnoid cysts, and intracranial lipomas. The translabyrinthine resection represents a common lateral approach to the petrous apex, internal auditory canal, and the cerebellopontine (CP) angle. The procedure does not allow for hearing preservation and is often chosen for tumors regardless of size where hearing preservation is not an issue.12 In this approach, removal of bone around the posterior aspect of the internal auditory canal and petrous apex results in exposure to both petrous apex and CP angle tumors. Further exposure may be gained with the transapical approach where the entire petrous bone is removed superiorly for wider exposure of the anterior CP angle. In addition to the expected absence of bone that is most apparent on CT, obliteration of the defect with fat is commonly seen on MRI ( Fig. 6.7 Axial (a) and sagittal (b) computed tomography images of a right canal wall-up mastoidectomy. The bony external auditory canal is maintained (b). Fig. 6.8 Axial (a) and coronal (b) computed tomography shows a canal wall-down mastoidectomy. There is resection of the posterior wall of the external auditory canal. This is also a radical mastoidectomy as the ossicles have been resected. Fig. 6.10 Axial T1-weighted postcontrast image (a) shows a large left vestibular schwannoma. (b) shows the patient following a translabyrinthine resection. The whole petrous bone including the ossicles and vestibular apparatus are removed and a fat graft has been placed into the surgical defect. When large lesions extend into the prepontine cistern, use of a transcochlear approach may be necessary. This technique is indicated most commonly in the setting of anterior-based meningiomas in the CP angle or at the clivus. Similar steps are involved as in a translabyrinthine resection, but the removal of the tympanic and petrous bones up to the clivus is required. The cochlea is removed, allowing for identification of the intrapetrous internal carotid artery as well as both horizontal and vertical segments of the facial nerve in the mastoid which can be transposed anteriorly or posteriorly as required. The external auditory canal is closed in a blind sac fashion and the Eustachian tube formally obliterated. Once again, the cavity is filled with fat and should not be confused with soft tissue representing recurrence. In summary, the translabyrinthine approach is used for access to the internal auditory canal and CP angle, for access to the trigeminal nerve in Meckel’s cave and for lesions involving the jugular foramen. By comparison, the transcochlear approach permits the former but also allows an approach for more anterior and medial lesions involving the clivus, intrapetrous carotid artery, and anterior middle cranial fossa if required. The transcochlear approach typically involves mobilization of the facial nerve with resultant weakness postoperatively being common. There is also greater risk for injury to the internal carotid artery. Surgery tends to be longer in duration. In a conservative (or retrolabyrinthine) petrosectomy, the posterior fossa dural plate is exposed inferiorly to the level of the jugular bulb preserving the otic capsule. The labyrinth forms the anterior limit of dissection. With this approach, the facial nerve is usually skeletonized and preserved in its vertical segment. Surgical exposure is slightly more limited but does allow for access to both posterior and middle cranial fossa dural plates. It is often combined with a temporal craniotomy that allows for retraction of the temporal lobe. Further exposure to the posterior fossa dura occurs with removal of bone posterior to the sigmoid sinus in the retrosigmoid (or suboccipital) area. In a lateral subtotal petrosectomy, the external auditory canal, tympanic membrane, malleus, and incus are removed. The ear canal skin is closed in a blind sac fashion and the Eustachian tube is formally obliterated. The term “lateral” in this context denotes that bone removal stops at but does not include the labyrinth. Hearing and labyrinthine function is preserved although the individual would have a maximum conductive hearing loss on the operated side. A total petrosectomy extends the dissection to the petrous apex as indicated for carcinoma involving the temporal bone. The goal would be to perform an en bloc resection if possible in order to avoid tumor spillage at surgery. The labyrinth, cochlea, and facial nerve would be removed. The surgical plane medially would traverse the internal auditory canal. Care would be taken not to injure the intrapetrous carotid artery. The infratemporal approach is used to expose the inferior part of the temporal bone including the parapharyngeal space internal carotid artery and internal jugular vein/jugular bulb. This approach allows for the removal of lesions from the CP angle to both the parapharyngeal and the jugulo-carotid spaces. Anterior transposition of the facial nerve is again required for direct surgical access in addition to an extension of the dissection into the neck for great vessel and lower cranial nerve identification. Depending on tumor extension, the infralabyrinthine portion of the petrous bone is removed and usually the external auditory canal. There is a known predisposition for cerebrospinal fluid (CSF) leakage, which is often offset by staging the surgery over two sessions.13 As previously mentioned a conservative (retrolabyrinthine) petrosectomy may be combined with a suboccipital craniotomy to afford a greater posterolateral approach to the CP angle, upper neck, and jugular foramen. The external auditory canal is kept intact along with the otic capsule. In addition, the parapharyngeal space will be seen to communicate through an inferior mastoid approach. A fat plug can usually be seen postoperatively, which is placed during the intra-extradural closure. Careful assessment of the postoperative internal carotid is imperative although it is unclear whether the limited carotid manipulation from this approach predisposes it to injury or whether this technique is chosen in cases where intrapetrous carotid canal involvement appears more likely.14 To visualize the anterior and posterior aspects of the petrous bone, a total transtemporal approach may be employed. Employing a sizable (6 × 5 mm) temporal craniotomy, bone is removed above the petrous bone revealing the temporal lobe dura. At surgery, the dura is elevated along posterior to anterior along the petrous bone. A transtentorial dural opening in addition to splitting of the tentorium cerebelli (and additional splitting of the tentorium cerebelli) can be performed if required to further elevate the temporal lobe for greater exposure. Alternatively, another direct approach to the CP angle can be made through a retrosigmoid (suboccipital or paracerebellar) craniotomy. This is typically recommended for tumors such as vestibular schwannomas or meningiomas which do not involve the distal end of the internal auditory canal or impinge the brainstem in those with serviceable hearing. Retrosigmoid bone is removed and the dura is opened to allow access to the posterior aspect of the temporal bone and CP angle. Retrolabyrinthine surgery is indicated as a hearing preservation procedure. Removal of the posterior bony portion of the porus acusticus allows for visualization of the intracanalicular facial, cochlear, and superior and inferior vestibular nerves. Hearing preservation rates may be present in 50 to 70% of carefully selected cases and are both tumor and extent dependent in the internal auditory canal.15 Despite cochlear nerve preservation, hearing is often lost from vascular injury to the labyrinthine arteries in the internal auditory canal. Though all techniques discussed will demonstrate expected patterns of bone removal, tissue seals around the internal auditory canal and cerebellar edema not infrequently seen following surgical retraction. The retrosigmoid approach has been well documented to have varying degrees of cerebellar edema and gliosis from surgical retraction not related to tumor pathology ( Dedicated T1-weighted MR, angled perpendicular to the dorsal aspect of the brainstem, can be used to visualize the facial nerve when detailed follow-up is required. The precise course of a transposed nerve can be followed or its sacrifice documented on this sequence. Facial nerve transposition is favorable to its surgical sacrifice for anatomic exposure whenever possible. On high-resolution T2-weighted MR or constructive interference in steady-state (CISS) sequences, the normal nerve can be seen as a hypointense linear structure extending from the brainstem to the internal auditory canal. It is normally surrounded by CSF and lies anterior to the vestibulocochlear nerve complex.16 Gadolinium is necessary to best visualize its tympanic and mastoid segments as well as the geniculate ganglion. While enhancement of these segments can reflect neoplastic involvement, caution is advised as postsurgical inflammation may impart the same appearance. In facial nerve reanimation surgery, a graft (often a sural or greater auricular nerve interposition graft) may be present for reconstruction of the nerve. Reanimated nerves may also enhance in the segments where the facial nerve does not typically do and should not be mistaken for recurrence. With careful patient selection, an increasing number of patients with sinonasal and anterior cranial fossa tumors are successfully being managed with a total endoscopic approach that spares the patient a lateral rhinotomy and the craniotomy portion of an open CFR.17 In such cases, margins of the mucosa, periorbital, and dura should be microscopically clear to ensure local control on par with traditional combined approaches. One method of reconstructing a skull base defect employs the Hadad–Bassagasteguy technique. In this technique, a nasoseptal mucoperiosteal flap supported by a nasoseptal vascular pedicle is used to seal the skull base defect (
6.1 Introduction
6.2 Posttreatment Fundamentals
6.3 Postsurgical Changes
6.3.1 Craniofacial Resection
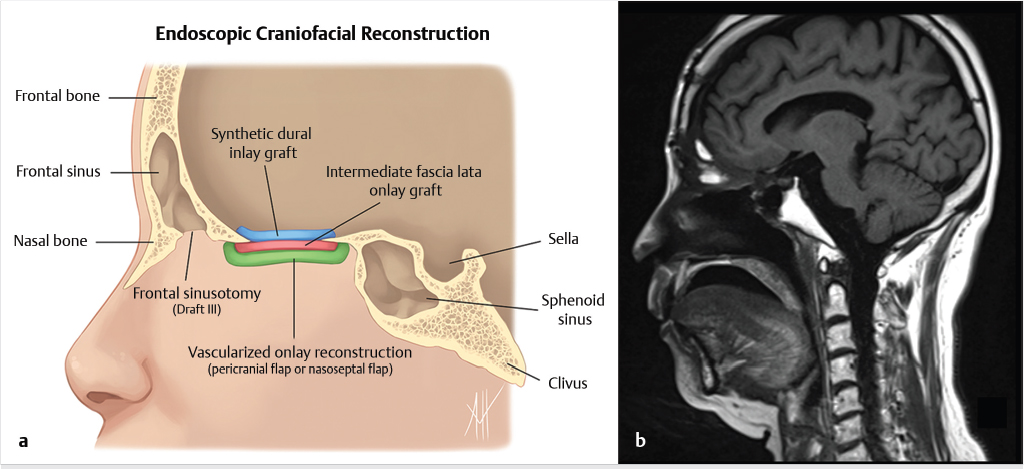
 Fig. 6.1). Cranialization involves removal of the posterior frontal sinus wall and frontal sinus mucosa. If the procedure is performed in a completely endoscopic manner (i.e., without a bicoronal craniotomy), a Draf III frontal sinusotomy procedure can be performed in place of frontal sinus cranialization. If a medial maxillectomy is performed as part of the resection, the normal fat density or signal in the pterygopalatine fossa will be replaced with a soft-tissue density scar. At our institution, the defect along the floor of the anterior cranial fossa is reconstructed with a synthetic duraplasty inlay graft, an optional intermediate fascia lata onlay graft, and then a vascularized onlay graft (consisting of either a pericranial flap or nasoseptal mucosal flap;
Fig. 6.1). Cranialization involves removal of the posterior frontal sinus wall and frontal sinus mucosa. If the procedure is performed in a completely endoscopic manner (i.e., without a bicoronal craniotomy), a Draf III frontal sinusotomy procedure can be performed in place of frontal sinus cranialization. If a medial maxillectomy is performed as part of the resection, the normal fat density or signal in the pterygopalatine fossa will be replaced with a soft-tissue density scar. At our institution, the defect along the floor of the anterior cranial fossa is reconstructed with a synthetic duraplasty inlay graft, an optional intermediate fascia lata onlay graft, and then a vascularized onlay graft (consisting of either a pericranial flap or nasoseptal mucosal flap;  Fig. 6.2). Anterior floor reconstruction will manifest as a soft-tissue density or signal that bridges across the bony defect. Variable degrees of enhancement will be present that may also bulge inferiorly into the nasoethmoid region (
Fig. 6.2). Anterior floor reconstruction will manifest as a soft-tissue density or signal that bridges across the bony defect. Variable degrees of enhancement will be present that may also bulge inferiorly into the nasoethmoid region ( Fig. 6.3). Pneumocephalus and extra-axial fluid collection is not uncommon in the early postoperative period. The mass effect and brain displacement secondary to the tumor as well as brain retraction and instrumentation during surgery may result in frontal lobe edema. This seems more pronounced in elderly patients. Accordingly when present, significant frontal lobe edema on MR should not be mistaken for recurrence or progression. A small collection of subdural blood may also be present, which again seems a more common finding in the elderly. With time, encephalomalacia may develop in the frontal lobes and would be manifested as increased T2 signal and volume loss (
Fig. 6.3). Pneumocephalus and extra-axial fluid collection is not uncommon in the early postoperative period. The mass effect and brain displacement secondary to the tumor as well as brain retraction and instrumentation during surgery may result in frontal lobe edema. This seems more pronounced in elderly patients. Accordingly when present, significant frontal lobe edema on MR should not be mistaken for recurrence or progression. A small collection of subdural blood may also be present, which again seems a more common finding in the elderly. With time, encephalomalacia may develop in the frontal lobes and would be manifested as increased T2 signal and volume loss ( Fig. 6.4).
Fig. 6.4).
 Fig. 6.2,
Fig. 6.2,  Fig. 6.4).
Fig. 6.4).
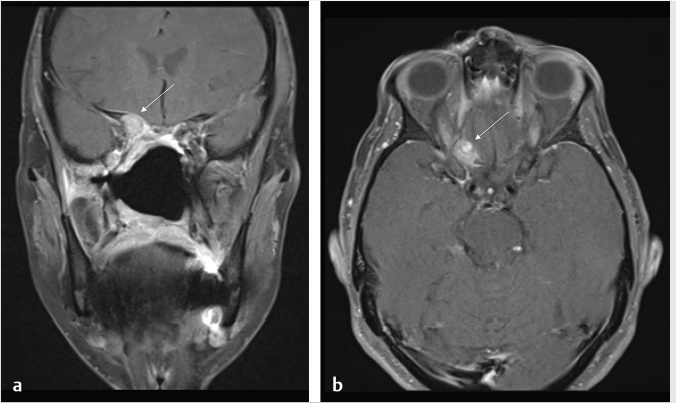
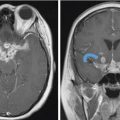
 Fig. 6.5,
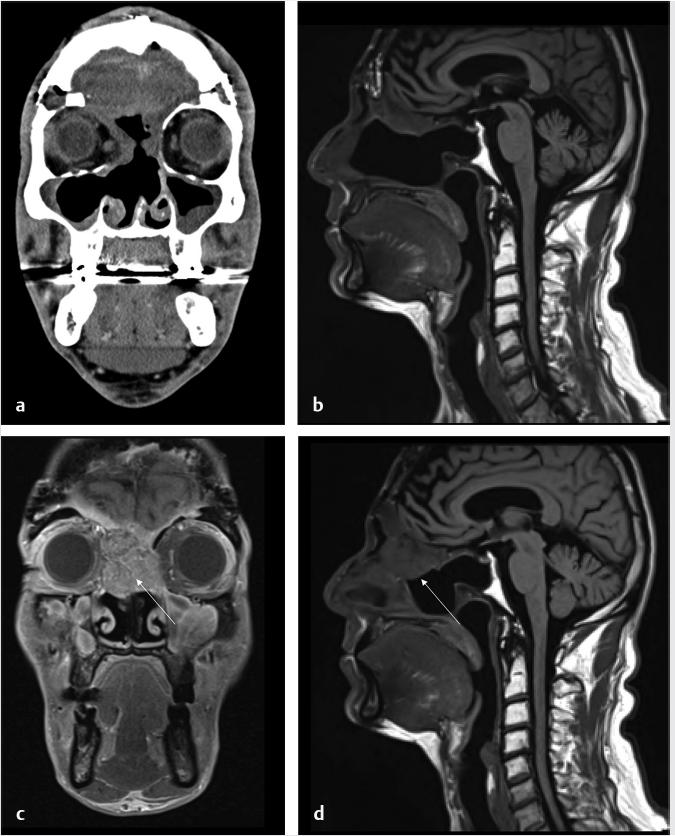
Fig. 6.5,  Fig. 6.6).8 Tumor recurrence often exerts mass effect locally and appears nodular or infiltrative in nature.
Fig. 6.6).8 Tumor recurrence often exerts mass effect locally and appears nodular or infiltrative in nature.
6.3.2 Lateral Skull Base Surgery
 Fig. 6.3) and is causing some mass effect on the frontal brain parenchyma.
Fig. 6.3) and is causing some mass effect on the frontal brain parenchyma.
6.3.3 Mastoidectomy
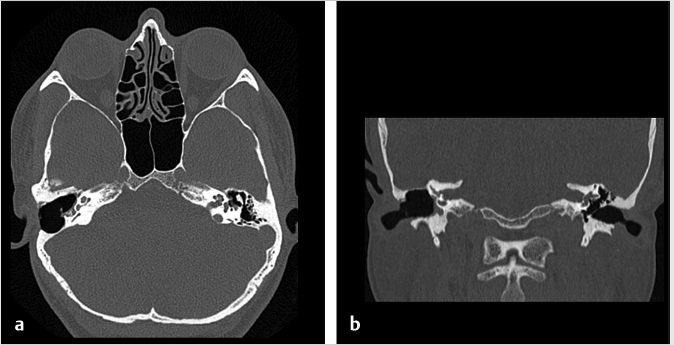
 Fig. 6.7). The surgically created mastoid bowl allows for a connection between the antrum and the epitympanum.10 In both procedures, the external auditory canal is preserved.
Fig. 6.7). The surgically created mastoid bowl allows for a connection between the antrum and the epitympanum.10 In both procedures, the external auditory canal is preserved.
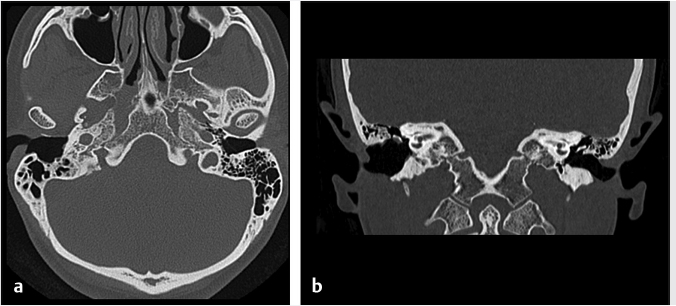
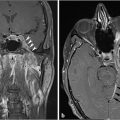
 Fig. 6.8). In a canal wall-down approach, the facial nerve within the mastoid may need to be sacrificed if involved by malignant disease. The procedure may involve a limited lateral temporal bone resection for adequate identification of the facial nerve.11 The scutum is removed in most cases. If disease involves the ossicular chain, then the ossicles (typically leaving the stapes intact) and tympanic membrane are removed. The facial nerve is skeletonized and may be sacrificed if necessary. The procedure of a radical mastoidectomy typically involves the removal of the tympanic membrane, malleus and incus with preservation of the stapes suprastructure, the external auditory canal, mastoid air cell exenteration, and formal obliteration of the Eustachian tube (
Fig. 6.8). In a canal wall-down approach, the facial nerve within the mastoid may need to be sacrificed if involved by malignant disease. The procedure may involve a limited lateral temporal bone resection for adequate identification of the facial nerve.11 The scutum is removed in most cases. If disease involves the ossicular chain, then the ossicles (typically leaving the stapes intact) and tympanic membrane are removed. The facial nerve is skeletonized and may be sacrificed if necessary. The procedure of a radical mastoidectomy typically involves the removal of the tympanic membrane, malleus and incus with preservation of the stapes suprastructure, the external auditory canal, mastoid air cell exenteration, and formal obliteration of the Eustachian tube ( Fig. 6.8). In a modified radical mastoidectomy, there is partial ossicular chain preservation (typically the handle of the malleus and the stapes suprastructure [the head of the malleus and incus are typically removed];
Fig. 6.8). In a modified radical mastoidectomy, there is partial ossicular chain preservation (typically the handle of the malleus and the stapes suprastructure [the head of the malleus and incus are typically removed];  Fig. 6.9). Hearing restoration surgery involving ossicular reconstruction or device implantation can be performed as indicated.
Fig. 6.9). Hearing restoration surgery involving ossicular reconstruction or device implantation can be performed as indicated.
6.3.4 Transpetrous Approaches
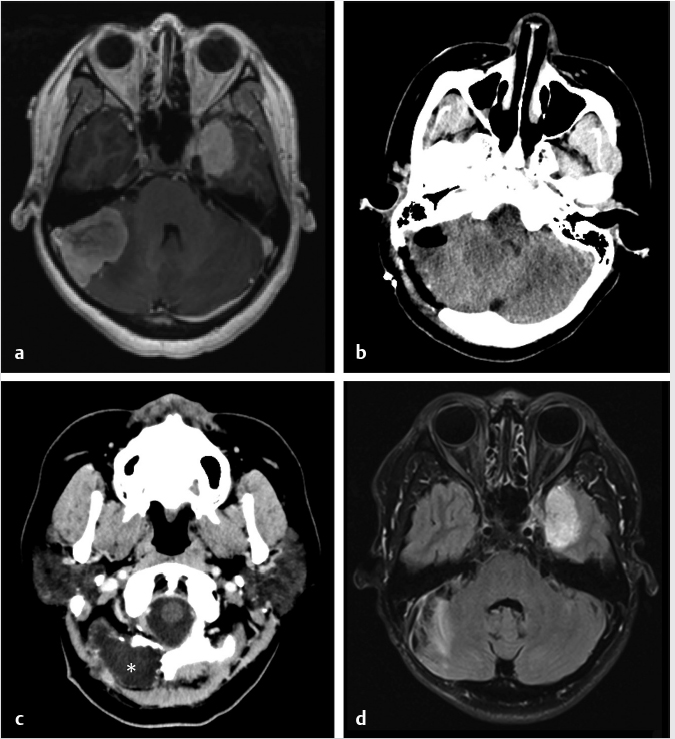
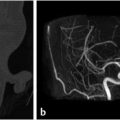
 Fig. 6.10). Gliosis in the adjacent cerebellar brain parenchymal from retraction at surgery may also be present.
Fig. 6.10). Gliosis in the adjacent cerebellar brain parenchymal from retraction at surgery may also be present.
6.3.5 Transtemporal and Retrosigmoid Approaches
 Fig. 6.11).
Fig. 6.11).
6.3.6 Endoscopic Surgery
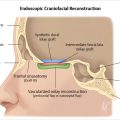
 Fig. 6.12). On T1- and T2-weighted imaging, these nasoseptal flaps appear isointense to brain. Immediately following surgery, a minority of these flaps may not enhance, but most do so. Most flaps also decrease in thickness on follow-up at 6 months. A small minority, however, increase in thickness. Neither characteristic can be used to predict flap failure, and the significance of these findings remains unknown. Enhancement on follow-up is likely the greatest predictor of flap viability with the caveat that some flaps may not enhance on early postoperative imaging but still remain viable.18,19 Free fat may be also noted between the defect and sinonasal cavity if placed intraoperatively.
Fig. 6.12). On T1- and T2-weighted imaging, these nasoseptal flaps appear isointense to brain. Immediately following surgery, a minority of these flaps may not enhance, but most do so. Most flaps also decrease in thickness on follow-up at 6 months. A small minority, however, increase in thickness. Neither characteristic can be used to predict flap failure, and the significance of these findings remains unknown. Enhancement on follow-up is likely the greatest predictor of flap viability with the caveat that some flaps may not enhance on early postoperative imaging but still remain viable.18,19 Free fat may be also noted between the defect and sinonasal cavity if placed intraoperatively.
Radiology Key
Fastest Radiology Insight Engine