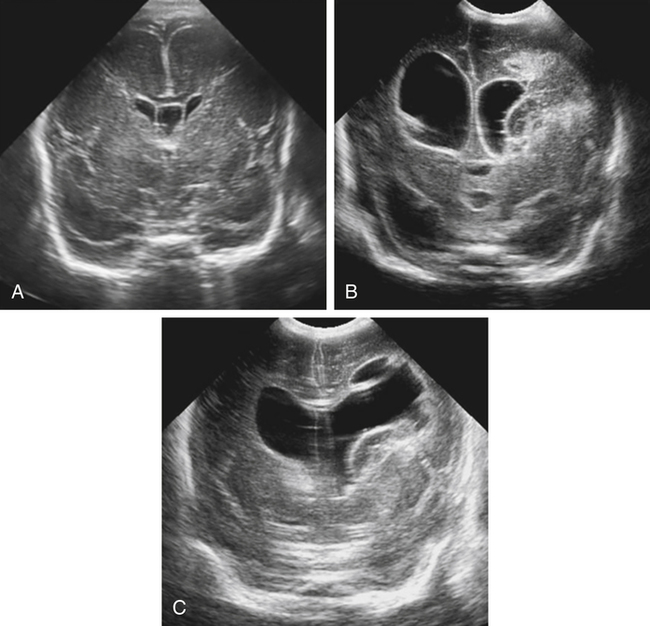
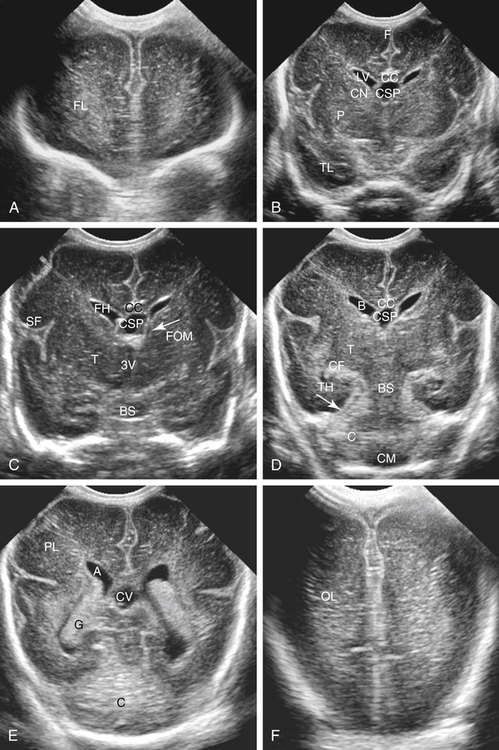
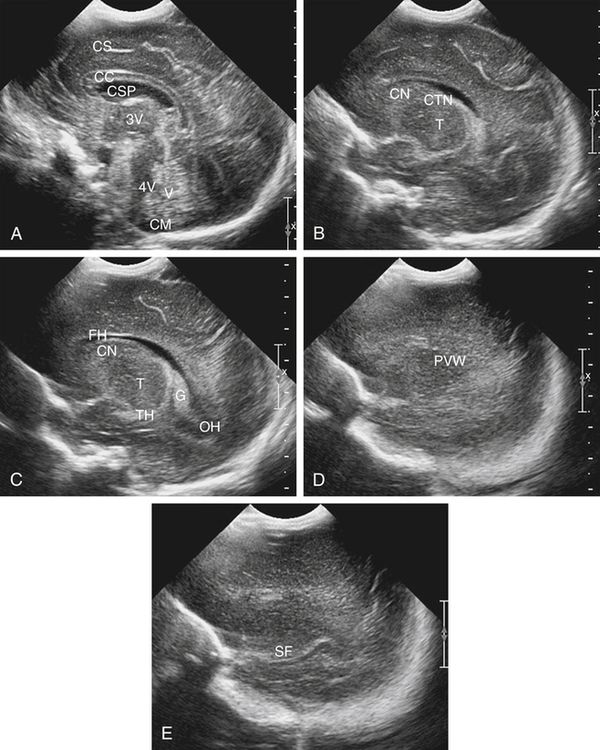
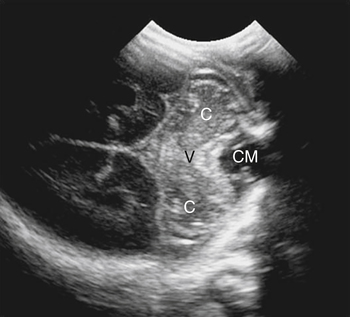
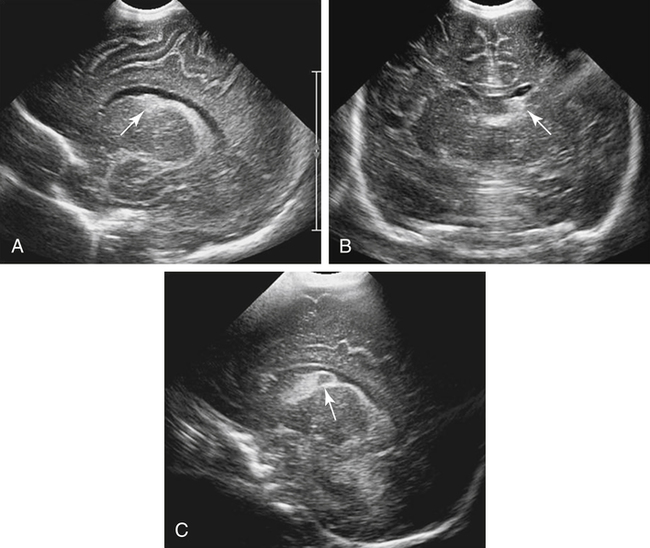
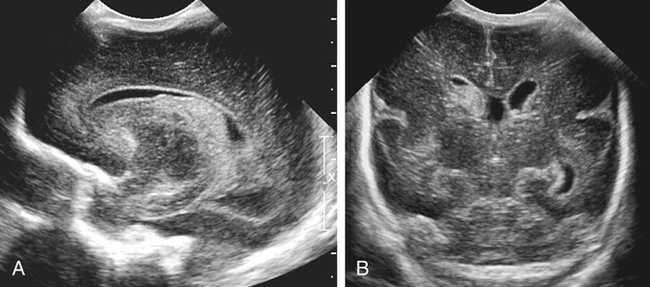
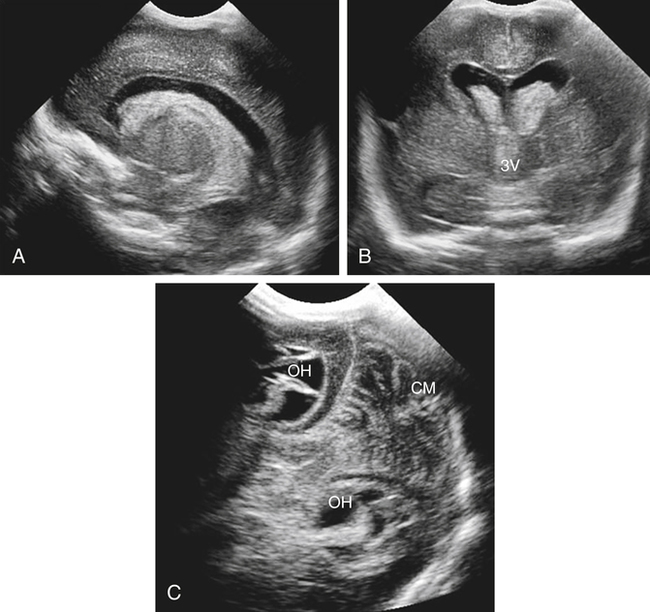
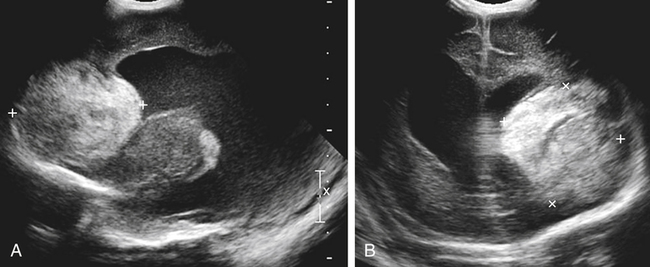
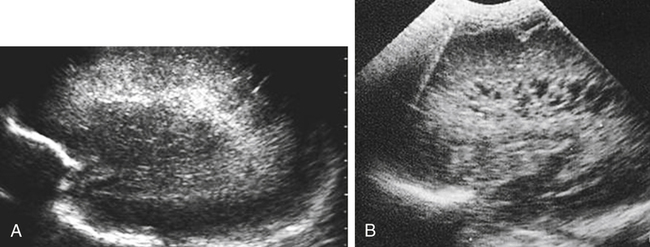
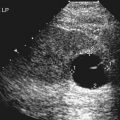
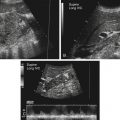
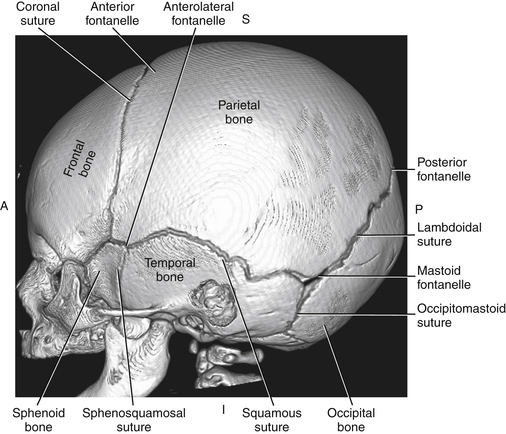
Imaging technique should include the selection of the highest frequency transducer possible without sacrifice of adequate penetration. Transducers of 7.5 to 10 MHz are typically selected for premature infants, and 5-MHz transducers are usually adequate for infants with larger heads. A small footprint transducer is needed for imaging through the small fontanelles. Linear array transducers are useful in evaluating superficial structures. Routine neonatal brain imaging is performed through the anterior fontanelle in the coronal, sagittal, and parasagittal planes. Parasagittal views are lateral to the midline (true sagittal) plane but are referred to as sagittal images in this chapter. The posterior and mastoid fontanelles provide additional acoustic windows for further evaluation of the posterior fossa (Fig. 32-2).1 C1: Frontal lobes of the brain, anterior to the frontal horns of the lateral ventricle (Fig. 32-3, A). C2: Frontal horns of lateral ventricles. Anatomy visualized includes falx, corpus callosum, cavum septi pellucidi (CSP), lateral ventricles, caudate nucleus, putamen, and temporal lobe (Fig. 32-3, B). C3: Lateral ventricles at the level of the third ventricle and foramen of Monro. Structures visualized include corpus callosum, CSP, frontal horns, foramen of Monro, third ventricle, thalami, sylvian fissure, and brainstem (Fig. 32-3, C). C4: Bodies of the lateral ventricles. Anatomy includes corpus callosum, CSP, choroid plexus in the body of the lateral ventricle, thalami, choroidal fissure, temporal horn, tentorium, cerebellum, and cisterna magna (Fig. 32-3, D). C5: Atria of the lateral ventricles. Anatomic structures include the parietal lobe, glomus of the choroid plexus, and cerebellum (Fig. 32-3, E). Sag ML: Midline image of the brain visualizing the cingulate sulcus, corpus callosum, CSP, third and fourth ventricles, cerebellar vermis, and cisterna magna (Fig. 32-4, A). Sag Left or Right 1: Lateral ventricle demonstrating the caudothalamic notch. Anatomy visualized includes the caudate nucleus, thalamus, and caudothalamic notch (Fig. 32-4, B). Sag Left or Right 2: Entire lateral ventricle showing the frontal, temporal, and occipital horns; glomus of the choroid plexus; caudate nucleus; and thalami (Fig. 32-4, C). Sag Left or Right 3: Periventricular white matter (Fig. 32-4, D). Sag Left or Right 4: Sylvian fissure containing the middle cerebral artery (Fig. 32-4, E). In addition, longitudinal placement of the transducer in the posterior fontanelle, with left and right angulation, allows assessment of the occipital horn for intraventricular hemorrhage. Placement of the transducer in the mastoid fontanelle, with superior to inferior angulation, aids in evaluating the cerebellum and cisterna magna (Fig. 32-5). Intracranial hemorrhage may be seen in premature and full-term infants and may be the sequela of an ischemic or hypoxic event, trauma, infarction, vascular malformation, or bleeding disorder. Germinal matrix–intraventricular hemorrhage is the most commonly diagnosed brain lesion in premature newborns.2 Complications of posthemorrhagic ventricular dilation and parenchymal injury may result in devastating neurologic effects. Cranial sonography is a reliable method for the screening of low-birth-weight (<1500g) or premature (<30 weeks’ gestational age) infants at increased risk for development of an intracranial hemorrhage. Approximately 80% of hemorrhages occur within the first 3 days of life, and screening for hemorrhage is usually performed at 1 week of age.3 Sonographic imaging for hemorrhage should identify the absence or presence of hemorrhage, ventricular enlargement, and parenchymal extension. Acute grade I hemorrhage is identified as a homogeneous, echogenic mass at the caudothalamic groove (Fig. 32-6, A and B). Subependymal hemorrhages are similar in echogenicity to the choroid plexus. Aging of a grade I hemorrhage may result in the formation of a subependymal cyst (Fig. 32-6, C). Grade II intraventricular hemorrhage can be diagnosed by evidence of echogenic material that partially or completely fills the ventricular cavity (Fig. 32-7). Development of ventriculomegaly related to the hemorrhage is classified as a grade III hemorrhage (Fig. 32-8). Grade IV or parenchymal hemorrhage is initially identified as an echogenic, homogeneous mass extending from the ventricle into the adjacent white matter (Fig. 32-9). Over time, the echogenicity of blood decreases, resulting in a cystic cavity extending from the ventricle to the parenchyma. This is known as a porencephalic cyst. Sonographic findings of PVL in the acute stage show bilateral areas of increased echogenicity in the white matter superior and lateral to the ventricular margins of the brain (Fig. 32-10, A). This finding may resolve or progress to cystic PVL. Cystic PVL typically develops 2 to 3 weeks after the insult and manifests as numerous, small cystic lesions paralleling the lateral ventricle of the brain (Fig. 32-10, B). These findings resolve within a few months, with resultant brain atrophy and subsequent ventriculomegaly.
Premature Birth
Rule Out Germinal Matrix Hemorrhage
Neonatal Head
Imaging Technique and Normal Sonographic Anatomy
Intracranial Hemorrhage
Sonographic Findings
Periventricular Leukomalacia
Sonographic Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Premature Birth: Rule Out Germinal Matrix Hemorrhage