Preoperative assessment with computed tomography (CT) is critical before transcatheter interventions for structural heart disease. CT provides information for device selection, device sizing, and vascular access approach. The interpreting radiologist must have knowledge of appropriate CT protocols, how and where to obtain the important measurements, and know additional imaging characteristics that are important to describe for optimal support of the interventionalist. CT is the modality of choice for pre-operative evaluation in patients undergoing transcatheter aortic valve replacement and left atrial appendage occlusion, and is also useful before transcatheter mitral valve replacement, which is an ongoing area of research.
Key points
- •
Pre-procedural computed tomography (CT) for structural heart disease is crucial to provide the interventionalist with information for vascular access approach, device selection, device sizing, and risk stratification.
- •
Proper orientation to the plane of the aortic annulus during systole on pre–transcatheter aortic valve replacement cardiac CT is important for accurate annular measurements for device sizing.
- •
Evaluation of left atrial appendage morphology, depth, and presence of thrombus are critical before intervention to assess feasibility of appendage occlusion and device sizing.
- •
Transcatheter mitral valve replacement device development is an area of ongoing investigation with preoperative assessment of the mitral annulus with cardiac CT playing an imperative role.
Background
Aortic stenosis (AS) is a common disease with prevalence increasing with age, and affecting 4% of the population older than 85. Although the disease has a spectrum of severity, untreated symptomatic severe AS carries a markedly high mortality. Historically, a major problem for a large subset of these patients was that treatment with open surgical valve replacement was contraindicated or risky because of multiple comorbidities. The development of transcatheter aortic valve replacement (TAVR) has given patients who would otherwise be exposed to high-risk surgery or limited to medical management a safe alternative for intervention. Recent trials have also expanded TAVR utilization to patients who are of intermediate and low risk for open surgery. These studies demonstrate that TAVR procedures perform equally well as surgery in these populations. Preoperative imaging with computed tomography (CT) has been a crucial step in the development and optimization of TAVR outcomes and is a requirement for presurgical planning for TAVR approach and device sizing.
Devices
There are 2 families of devices with Food and Drug Administration (FDA) approval for use in TAVR: the Edwards Sapien valve (Edwards Lifesciences, Irvine, CA) and the Medtronic CoreValve (Medtronic, Minneapolis, MN). Both devices have undergone several device updates since initial introduction. The major innovations include a reduction in the diameter of the delivery sheath and introducing skirts to reduce paravalvular leak (PVL). The additional major device characteristics, however, have remained unchanged and are presented in Table 1 . Examples of these TAVR devices are shown in Fig. 1 .
| Sapien Valve | CoreValve | |
|---|---|---|
| Sizing method | Area | Perimeter |
| Expansion technique | Balloon-expandable | Self-expandable |
| Material | Bovine pericardial tissue | Porcine pericardial tissue |
| Components | Polyethylene terephthalate skirt at the base of Sapien III, which decreases PVL | Covered segment with flared end |
| Sizes, mm | 20, 23, 26, 29 | 23, 26, 29, 31 |
| Valve location | Annular | Supra-annular |
| Trial showing efficacy/noninferiority to SAVR | PARTNER A, B, II, III | CoreValve Extreme Risk and High Risk trials; SURTAVI, CoreValve Low Risk trial |
| Primary method of delivery | Transfemoral access | Transfemoral access |

Imaging
Modalities
CT imaging provides a comprehensive assessment pre-TAVR. The first trials evaluating the efficacy of TAVR used echocardiography (echo) for device sizing; however, CT has been shown to have multiple advantages over echo pre-TAVR. , Several studies have shown that the rate of PVL is lower when CT measurements are used for device sizing as compared with echo. This can be explained by the fact that 3-dimensional (3D) volumetric CT datasets allow visualization of the entire aortic annulus, permitting accurate area and perimeter measurements, whereas by 2D-echo, annular size is calculated from a single diameter measurement obtained from the parasternal long axis view, which underestimates true annular size. Underestimation of annular measurements leads to undersizing and PVL, which has significant implications, because PVL has been shown to increase mortality in TAVR patients.
The other major role of CT for preoperative planning in TAVR is identifying the optimal route for vascular access. Most commonly, the TAVR device is introduced using a delivery sheath via the femoral arteries. Marked atherosclerotic disease, small vessel size, and/or vessel tortuosity may interfere with the ability of the interventionalist to access the femoral vessels or increase the risk of vascular complications, such as vessel rupture or dissection. CT angiography is used to image the entirety of the aorta, the proximal great vessels, and the ilio-femoral vasculature, allowing the interventionalist to plan for the safest approach. Vessel diameters of 6 mm or greater are typically sufficient for the newest generation devices. In addition to vessel size, calcification and tortuosity are assessed. Severe tortuosity, particularly if the tortuous vessel is stiff due to calcification, could make it very difficult to deliver the device sheath. If the characteristics of the femoral arteries do not allow viable vascular access, then a direct surgical cut-down to the iliac arteries or subclavian access are the preferred secondary options. In patients with severe vascular disease of both the iliacs and subclavians, direct access into the ascending aorta and transapical access through the apex of the left ventricle have been used.
In some patients with anaphylaxis to iodinated contrast or severe renal dysfunction, CT angiography may not be an option. In these cases, noncontrast MR imaging of the heart, with cine image of the left ventricular outflow tract (LVOT) and aortic valve as well as noncontrast 3D MR angiography of the thoracic aorta can be performed. Evaluation of the abdominal and pelvic vasculature could be performed with noncontrast MR angiography or could be performed with noncontrast CT of the abdomen and pelvis to evaluate external vessel diameters.
Computed Tomography Parameters
Recommended parameters and protocol information for pre-TAVR CT are included in Table 2 . CT should be performed on a multidetector, wide-area, high-pitch scanner, commonly a 256-slice or 320-slice scanner. CT protocol should include an electrocardiography (ECG) gated cardiac CT followed by a nongated CT angiography chest, abdomen, pelvis with iliofemoral runoff. Thin-slice reconstructions allow for high-resolution images when orienting to the annulus. The limiting factor is access, as not all institutions have this type of scanner of available and instead use a 64-slice scanner. An alternative protocol using a 64-slice scanner is presented in Table 3 . All imaging should be done supine.
| Study | ECG Gating | Contrast Dose | ROI Location | ROI Trigger Threshold | FOV | Tube Potential | Recons |
|---|---|---|---|---|---|---|---|
| Cardiac CT | Retrospective or prospective | 80–120 mL | Mid ascending aorta | 250 HU | Heart | 100–120 kV | Volumetric 0.5–0.75 mm |
| CTA CAP runoff | Nongated, immediately following cardiac CT | No additional contrast | None | None | Open | 100–120 kV | Volumetric0.5–1.0 mm |
| Study | ECG Gating | Contrast Dose | ROI Location | ROI Trigger Threshold | FOV | Tube Potential | Recons |
|---|---|---|---|---|---|---|---|
| Cardiac CT | Retrospective or prospective | 1st injection of 80 mL | Mid ascending aorta | 90–125 HU | Heart | 100–120 kV | Iterative 0.5–0.75 mm |
| CTA CAP runoff | Nongated | 2nd injection of 50 mL | Descending aorta | 90–125 HU | Open | 100–120 kV | Iterative 0.5–1 mm |
Contrast
Iodinated contrast is needed for thorough assessment of the heart and vascular anatomy. Contrast should be injected through a wide-bore intravenous catheter, typically in the antecubital fossa, at a rapid rate of injection of 5 to 7 mL/s, followed by a 40-mL saline flush delivered at the same rate. The saline flush is important, as it helps clear contrast in the ipsilateral subclavian vein, which otherwise may produce streak artifact limiting evaluation of the ipsilateral subclavian artery. Contrast dose, region of interest (ROI) location, and ROI trigger thresholds are presented in Tables 2 and 3 . Contrast dose can be reduced depending on glomerular filtration rate.
Electrocardiogram Gating
ECG gating is a vital part of the CT because it helps obtain the best-quality images possible of the heart and aortic root. Retrospective gating acquires images throughout multiple cardiac cycles and is generally recommended when available. Although retrospective gating results in increased radiation exposure as opposed to prospective (step and shoot) gating, it has the advantage of allowing for ECG editing in patients with unexpected arrhythmias, often salvaging an otherwise limited examination. For some scanners that do not permit use of retrospective gating, prospective gating with a wide imaging window can be used. Use of dose modulation helps to limit radiation exposure, only using full radiation dose for acquisition of images during the portion of the cardiac cycle that is of interest. For pre-TAVR CT, this is typically 30% to 80% of the R-R interval; however, can be narrowed to 20% to 40% of the R-R interval at institutions where only systolic-phase images are acquired. Imaging of the systolic-phase pre-TAVR is crucial, as this is when the aortic root is maximally distended and is the phase for which annular measurements should be obtained. Annular measurements in a phase other than maximal distention risks device undersizing. In some institutions, annular measurements are obtained in the systolic phase selected by the scanner as the most motion free (“best systolic”) and the measurements of the sinuses and coronary heights are obtained in the most motion-free diastolic phase. Other institutions use systolic and diastolic measurements at a set phase of the cardiac cycle (commonly 20% and 70%) for measurements. Annular measurements must be obtained in systole; however, there is no guideline recommendation for measurements of the sinuses of Valsalva and coronary heights, which is up to local institutional preference.
Troubleshooting with Computed Tomography
There are several conditions that can limit CT quality, which limits assessment pre-TAVR, particularly for device sizing. Examples of these issues with proposed solutions are presented in Table 4 .
| Scenario | CT Limitation | Proposed Solution |
|---|---|---|
| Cardiac arrhythmias | Image misregistration | ECG editing, prospective systolic gating |
| Suboptimal contrast bolus (due to contrast extravasation, IV size, and/or poor patient hemodynamics) | Poor contrast opacification or mistimed examination | Use of large-bore IV, reduce ROI trigger threshold, divide examination into 2 parts, low kVp imaging |
| Poor patient breath-holding ability | Motion artifact | Reduce coverage of cardiac CT to focus on aortic valve, reducing scan length |
Pre–transcatheter Aortic Valve Replacement Assessment
The following measurements/characteristics are standardly reported on pre-TAVR CT:
- •
Aortic valve morphology (tricuspid vs bicuspid)
- •
Maximum and minimum aortic annulus diameter
- •
Annulus mean diameter
- •
Annulus perimeter and area
- •
Ascending aorta diameter 40 mm above the annulus
- •
Sinus of Valsalva diameters and heights
- •
Sinotubular junction diameter
- •
Ostial heights of the main coronary arteries
- •
Ideal fluoroscopic angle (C-arm angulation)
- •
Iliofemoral artery minimum luminal diameter
- •
Presence and severity of annular calcification
Aortic Annulus
Although all of the measurements listed in the preceding section play an important role in TAVR, aortic annular measurements are the most critical, as these will influence device sizing. To achieve the proper annulus orientation, a double-oblique method is used to demonstrate the plane of the most basal attachment points of the 3 aortic cusps, known as the basal ring, which is the correct plane for annular measurements. An example of orienting to the aortic annulus is shown in Fig. 2 , with annular measurements in Fig. 3 .


Annular measurements can be done manually, semiautomatically, or with automated software from one of multiple vendors. Specifically, perimeter and area can be measured using freehand contour, polygon tools, attenuation-based contour detection, and cubic spline interpolation; however, it is important to note that methods producing straight or jagged lines risk inaccurate measurements, as the annulus is smooth and ovoid. Smoothing tools should be applied in these situations to improve precision. Use of contouring tools is important, and manual caliper measurements should not be used for overall annular measurements. Contouring in the presence of annular calcifications can be challenging for accurate sizing. At our institution, the contour is drawn bisecting the calcium.
The aortic annulus is a dynamic structure, with maximum dimensions during systole. The annular measurements must be performed during systole to minimize the risk of device undersizing. Both area and perimeter are reported, as the Sapien valve uses area for device sizing, whereas CoreValve sizing is based on perimeter.
Aortic Valve Morphology
The role of TAVR in patients with a bicuspid aortic valve has been an area of controversy. Patients with bicuspid valves were initially excluded from early major device trials, and most data on the role of TAVR in patients with bicuspid valves stems from nonrandomized registry data. Although some studies have shown similar outcomes between patients with tricuspid and bicuspid aortic valves, others have shown higher rates of PVL and lower rates of device success in patients with a bicuspid aortic valve. Evaluating bicuspid aortic valves before the TAVR procedure can be difficult. Annular measurements can be more challenging, particularly in patients with a valve morphology containing 2 hinge points on the annulus. Care must be taken to perform annular measurements at the base of the valve leaflet insertions. More information on characterization of bicuspid valve morphology based on the number/presence of commissures and raphes can be found in Jilaihawi and colleagues.
Coronary Ostia Heights
The Sapien valve has covered metal struts seated at the level of the aortic annulus. The coronary ostia height measurement (shown in Fig. 4 ) is important because of the potential risk of these struts obstructing the coronary artery, following placement leading to reduced myocardial perfusion. Coronary occlusion, although rare, can occur in fewer than 1% of TAVR procedures, and has been more frequently reported with the Edward Sapien device. , An ostial height less than 12 mm does not necessarily preclude TAVR placement; however, increases the risk of coronary artery obstruction. , The CoreValve device design is associated with a lower risk of coronary obstruction due to an uncovered portion that sits above the level of the valve allowing for retrograde blood flow around the covered portion and into the coronary ostia.

Sinus of Valsalva
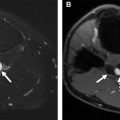
The Sinus of Valsalva (SOV) is the anatomic dilatation of the aortic root superior to the annulus and leaflets. Although size of the SOV varies throughout the cardiac cycle, there is no consensus if SOV measurements should be done during systole or diastole. This structure is also relevant to coronary artery obstruction because during TAVR the diseased leaflets are pushed superiorly and laterally by the transcatheter heart valve (THV) into the SOV. There is potential for ostial obstruction in patients with shallow sinuses, when bulky leaflet calcification is displaced by the device over the ostia. SOV diameters ≥30 mm are desired to minimize risk of coronary artery obstruction. Examples of SOV heights are shown in Fig. 5 .

Sinotubular Junction
The sinotubular junction (STJ) is the demarcation between the SOV and the more tubular aortic configuration, with the STJ height defined as the measurement from the annulus to the most inferior point of the STJ. The STJ and annulus are commonly not parallel, which is why the measurement is specifically to the most inferior aspect of the STJ. The STJ diameter should be measured using a transverse, double-oblique plane aligned with the STJ. This measurement is essential, particularly when a balloon-expandable device is being considered, because if the STJ height is low, an STJ diameter less than device diameter increases the risk for STJ injury.
Calcification
Calcium assessment of the aortic root is relevant because evidence suggests that severe subannular calcification is associated with an increased risk of annular rupture and PVL. , In addition, calcification of the leaflets and STJ can also affect device deployment. Currently, both qualitative and quantitative methods for assessing degree of annular and subannular calcification are performed. A qualitative calcium assessment scores the calcification ranging from mild-moderate-severe. Quantitative assessment of aortic leaflet calcification can be helpful for evaluation of patients for whom there is equivocal echocardiographic data for AS severity. Agatston scoring of the atrioventricular leaflet can be used to differentiate severe AS from moderate AS in patients with conflicting echo measurements, with supporting evidence from Pawade and colleagues showing that Agatston score is a powerful discriminator of severe AS.
Ideal Fluoroscopic Angle (C-Arm Angulation)
The ideal fluoroscopic angle describes the optimal angle of device deployment when under fluoroscopy and is acquired with software analysis of pre-TAVR CT. Use of this calculation has multiple advantages, as it may reduce fluoroscopic time, thereby reducing radiation exposure and also reducing contrast load. , Due to the limited motion of the C-arm, providing alternative angles is recommended when the cranial or caudal angle is >25°. Patient positing of the chest needs to be the same during CT and fluoroscopy for the derived calculation to be applicable, which can make reproducibility challenging.
Device Oversizing
Just as undersizing poses risks to the patient, device oversizing risks annular rupture, which is associated with a high mortality. Risk of annular rupture increases as degree of oversizing increases, but is particularly high when oversizing is greater than 20%. Although some degree of oversizing is desired for the THV, the degree of ideal oversizing is device-specific. Device oversizing is calculated using the following equation: Oversizing [%] = (THV nominal measurement/annular measurement − 1) × 100. Device oversizing is typically not addressed in the radiographic report but rather a decision that is made by the operator in collaboration with device representatives.
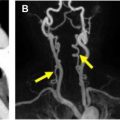
Iliofemoral Artery Minimum Luminal Diameter
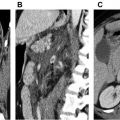
Vessel luminal diameter (shown in Fig. 6 ) is key to ensure that vascular access of the iliofemoral vessels with the device delivery sheath is possible. Measurements should be performed in a plane perpendicular to vessel trajectory using curved planar reconstructions from an automated software analysis tool. A qualitative assessment of atherosclerotic calcification also should be done.