- •
Preoperative risk stratification should be a comprehensive assessment with careful evaluation of active cardiac conditions, clinical risk factors, and planned surgical intervention.
- •
Noninvasive ischemic evaluation should be pursued for those with high-risk features in which the results will have an impact on management.
- •
Radionuclide imaging with SPECT is an excellent tool with diagnostic and prognostic value in the preoperative setting.
- •
Alternative imaging modalities can provide useful information and should be considered to assess specific conditions such as valvular heart disease.
- •
Test selection should be based on clinical utility, contraindications, and local expertise.
- •
The decision to perform coronary revascularization or alter medical therapy before noncardiac surgery should be made on an individualized basis.
Introduction
Major adverse cardiovascular events (MACE), such as myocardial infarction (MI) and stroke, are feared complications in the perioperative period. They account for up to a third of all perioperative deaths and are associated with higher costs and increased long-term mortality. Many patients undergoing noncardiac surgery have underlying coronary artery disease (CAD), which may be undiagnosed at the time of surgery. Given the fact that over 300 million surgeries are performed every year, a significant portion of patients undergoing noncardiac surgery are at increased risk for MACE. Thus a comprehensive cardiac risk assessment is vital to identify patients at significant risk for perioperative MACE who may benefit from alternative procedures or risk-modification strategies before surgery.
Goals of the perioperative cardiac evaluation
Preoperative cardiac risk assessment is an ever-evolving area, given changes in surgical/anesthetic strategies and improved medical therapy. It is a comprehensive evaluation that includes an assessment of the risk level of surgery, a thorough history and physical examination, and an assessment of the patient’s functional status. It can have a meaningful impact on patient care in several ways. First, it permits informed decision making by the patient and family. If patients are deemed to be at elevated risk, noninvasive testing may be conducted to evaluate the presence, extent, and severity of CAD. The results of testing may lead to cancellation or modification of the planned procedure, alterations of perioperative care and monitoring, or consideration of coronary revascularization, all with the goal of potentially improving outcomes. When considering additional testing, careful patient evaluation incorporating appropriate patient and test selection is necessary for optimal evaluation. Nuclear cardiology imaging techniques, such as radionuclide myocardial perfusion imaging (MPI), form an important part of the repertoire of currently available noninvasive stress testing modalities to evaluate for CAD. In this chapter, we will discuss the evolution and role of radionuclide MPI in preoperative cardiac risk stratification.
Historic background of the use of radionuclide MPI for preoperative risk stratification
The foundational work establishing a role for nuclear imaging techniques for preoperative assessment of cardiac risk was mainly conducted in the 1980s and 1990s. In that era, perioperative morbidity and mortality from MACE were deemed to be unacceptably high despite medical advances. Furthermore, the available indices of perioperative cardiac risk at the time were mainly derived from surgical cohorts with a low prevalence of CAD. It was increasingly recognized that these indices were poorly predictive of MACE in surgical cohorts with a high prevalence of CAD, such as in patients undergoing vascular surgeries. Lastly, although exercise stress testing was available as a noninvasive testing tool, a significant proportion of patients (such as those with peripheral vascular disease) were unable to exercise adequately. Therefore there was a clear need for the development of newer strategies for adequate preoperative cardiac evaluation, especially in patients with a high prevalence of CAD. Given its suitability for the assessment of myocardial perfusion, function, and ischemia, nuclear cardiology was well poised to fill this need. Additionally, radionuclide MPI may be combined with pharmacologic stress to assess patients unable to perform exercise testing.
In 1985, Boucher et al. were the first to use radionuclide imaging for preoperative cardiac risk assessment. Dipyridamole 201 thallium MPI was performed in patients with known CAD before peripheral vascular surgery, and the authors observed that the presence of reversible defects was predictive of adverse outcomes and that patients with fixed defects did not have any adverse outcomes. In 1987, Leppo et al. observed that, compared with clinical and electrocardiographic parameters, myocardial ischemia, as demonstrated on MPI, was a superior predictor of perioperative MI or death. Since these initial landmark studies, there has been a breadth of work that has informed the clinical guidelines set forth by several clinical societies ( Table 10.1 ). Nevertheless, to optimize the use of this technique, clinical risk assessment should be performed.
| Study | Number of patients (N) | Patients with ischemia demonstrated (%) | Perioperative adverse events (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| Boucher et al (1985) | 48 | 33 | 6 | 19 | 100 |
| Cutler and Leppo (1987) | 42 | 47 | 10 | 20 | 100 |
| Eagle et al (1989) | 200 | 41 | 8 | 16 | 98 |
| Younis et al (1990) | 111 | 36 | 7 | 15 | 100 |
| Hendel et al (1992) | 327 | 51 | 9 | 14 | 99 |
| Lette et al. (1992) | 355 | 45 | 8 | 17 | 99 |
| Brown et al (1993) | 231 | 33 | 5 | 13 | 99 |
| Koutelou et al (1995) | 106 | 44 | 3 | 6 | 100 |
| Stratmann et al (1996) | 229 | 29 | 5 | 6 | 99 |
| Huang et al (1998) | 106 | 36 | 5 | 13 | 100 |
| Cohen et al (2003) | 153 | 31 | 10 | 4 | 100 |
| Harafuji et al (2005) | 302 | 30 | 3 | 2 | 100 |
Risk assessment
There are numerous methods proposed for the cardiac risk assessment of patients undergoing noncardiac surgery, usually starting with an assessment of the level of urgency of surgery. Because emergency surgery is time-sensitive, it does not require additional testing, which could delay potentially life-saving interventions. Moreover, patients with active cardiac conditions, such as acute heart failure or acute coronary syndromes, need to be appropriately treated and stabilized before undergoing an elective surgical procedure ( Table 10.2 ). Once it has been determined that the surgical procedure is not emergent and there are no active cardiac conditions, a comprehensive cardiac risk evaluation can proceed. This commonly begins with an evaluation of surgical risk factors (i.e., risk level of surgery) and patient-specific risk factors (such as comorbidities and functional capacity). This evaluation represents the cornerstone when deciding whether to proceed with further cardiac testing ( Fig. 10.1 ).
| Condition | Examples |
|---|---|
| Unstable coronary syndromes | Unstable or severe angina (CCS Class III or IV), Recent MI |
| Decompensated heart failure | NYHA Class IV Worsening or new-onset HF |
| Significant arrhythmias | High-grade AV block Mobitz II AV block Third-degree AV block Symptomatic ventricular arrhythmia Supraventricular arrhythmias (including atrial fibrillation) with uncontrolled ventricular rate (>100 beats/min at rest) Symptomatic bradycardia Newly recognized ventricular tachycardia |
| Severe valvular disease | Severe AS (mean PG >40 mm Hg, AVA <1.0 cm 2 , or symptomatic) Severe MS (progressive dyspnea on exertion, exertional presyncope, or HF) |

Surgical factors: Low, intermediate, or high risk and special populations
Various factors intrinsic to the surgery influence a given patient’s perioperative cardiac risk. These include the urgency of surgery, invasiveness, duration of procedure, and perioperative blood loss and fluid shifts. There are various proposed methods to classify surgical procedures in terms of perioperative risk. A commonly used system is one developed by Glance et al. that classifies procedures as low, moderate, or high risk based on a 30-day risk for cardiovascular death and MI, where the risk levels were less than 1% in the low-risk group, 1% to 5% in the intermediate-risk group, and greater than 5% in the high-risk group, respectively. This method is commonly used and has been adopted by the 2014 European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA) guidelines. Low-risk procedures include superficial procedures, such as cataract surgery and plastic surgeries, whereas high-risk procedures include major invasive procedures, such as vascular and abdominal procedures ( Table 10.3 ). In contrast, the American College of Cardiology/American Heart Association (ACC/AHA) classify the risk level as low or elevated risk. Low-risk procedures are defined as those with a risk for MACE of less than 1% and include procedures such as cataract surgery or superficial plastic surgery. , All other procedures are classified as elevated risk. The ACC/AHA recommend determination of the risk for MACE using a combination of commonly used and validated risk prediction models discussed further in this section.
| Low Risk (<1%) | Intermediate Risk (1%–5 %) | High Risk (>5%) |
|---|---|---|
| Breast | Cholecystectomy | Major vascular surgery (including aortic) |
| Dental | Endovascular aneurysm repair | Liver transplant |
| Superficial surgery (e.g., lymph node excision) | Renal transplant | Esophagectomy |
| Endocrine: thyroid | Major orthopedic (i.e., hip and spine surgery) | Pneumonectomy |
| Minor orthopedic (e.g., arthroscopy) | Symptomatic carotid surgery/stenting | Exploratory laparotomy |
| Asymptomatic carotid surgery/stenting | Splenectomy | Perforated bowel repair |
| Eye (e.g., cataract) | Peripheral arterial angioplasty |
A unique type of surgery is organ transplantation, which is increasingly being performed across the world. The majority of currently available data on pretransplantation risk assessment is on renal and liver transplantation, representing a special population with unique characteristics. CAD is a common comorbidity in patients undergoing organ transplantation, especially of the kidney, and has significant implications for the perioperative course of these patients. It has been estimated that more than 50% of patients undergoing renal transplantation and approximately 2% to 30% of patients undergoing liver transplantation may have underlying CAD. , Transplant patients are at risk for perioperative cardiac complications not only because of their underlying CAD but also because of the unique nature of transplant surgery itself. Most solid organ transplant surgeries are major abdominal surgeries, with several factors that are significant stressors on the cardiovascular system. These include but are not limited to extended duration of general anesthesia, large fluid shifts, and potentially massive blood loss. As such, these patients form a special cohort with a unique surgical risk profile and need special attention during the risk evaluation process. Both liver and renal transplantation are considered to be elevated-risk procedures.
There is also emerging interest in the optimal cardiac risk assessment of patients undergoing hematopoietic stem cell transplantation (HCT), especially because survival after HCT continues to increase thanks to advancements in the field. Patients undergoing HCT may be at elevated-risk for cardiac complications compared with the general population. A feared cardiac complication after HCT is new-onset heart failure because the preparative chemotherapy regimens used to ablate the marrow before HCT may include cardiotoxic compounds, such as anthracyclines. As the selection criteria for HCT become less restrictive because of advancements in the field, the prevalence of cardiovascular risk factors and underlying CAD may increase in these patients.
Patient factors: Individual risk factors and functional capacity
Once the level of surgical risk is established, the next step among patients at elevated risk is to consider important patient-specific risk factors that are known to influence perioperative outcomes. These include demographics (such as age), chronic conditions, and functional status. Increased age is associated with an increased risk for complications because of the increasing prevalence of CAD, diabetes mellitus, and cerebrovascular disease as patients age. , Bateman et al. found an increased incidence of perioperative acute ischemic stroke in patients older than 65 years of age undergoing common surgical procedures. Along with older age, frailty, as defined by cognitive impairment and impairments in ability to perform instrumental activities of daily living, is associated with an increased risk for complications as well.
Cardiovascular risk factors that increase the risk of perioperative MACE include known CAD, heart failure, and valvular heart disease. Elective surgery should be delayed in the presence of any active cardiac conditions (see Table 10.2 ), such as acute coronary syndromes, decompensated heart failure, uncontrolled arrhythmias, and symptomatic valvular heart disease, until these issues are addressed appropriately. Moreover, a history of recent MI (<60 days) has been shown to correlate with increased incidence of perioperative MACE, including stroke. Heart failure poses a significant risk for perioperative complications and has significant implications as the prevalence of heart failure continues to increase. Left ventricular ejection fraction (LVEF) has a substantial impact on perioperative risk because severely decreased LVEF (<30%) is associated with reduced survival in the perioperative period. Patients with heart failure with preserved ejection fraction tend to do better than their counterparts with reduced ejection fraction but not as well as patients without heart failure. Valvular heart disease, obesity, and atrial fibrillation are also known predictors of adverse perioperative outcomes.
Functional status significantly affects perioperative risk. A common measure of functional capacity is in terms of metabolic equivalent tasks (METs), where 1 MET is defined as 3.5 mL of O 2 uptake per kilogram per minute, which is also the resting oxygen requirement of the average 40-year-old, 70-kg man. , Patients with poor functional status (<4 METs), such as those who are unable to climb up a flight of stairs, are at higher risk for perioperative cardiac complications compared with patients with normal functional status. , In some patients, such as those with advanced orthopedic conditions like knee arthritis, it is not useful to assess functional capacity using subjective tests like assessment of METs. In these patients, more objective testing, such as the Duke Activity Status Index (DASI) ( Table 10.4 ) or cardiopulmonary exercise testing (CPET) are useful alternatives. The most comprehensive assessment of overall functional capacity is CPET because it can aid in differentiating between cardiopulmonary and noncardiopulmonary causes of dyspnea and exercise intolerance and provide an objective assessment of disease severity and patients’ functional/exercise capacity. , Furthermore, most patients who otherwise have limited exercise capacity because of orthopedic reasons, such as severe knee arthritis, may be able to adequately complete CPET testing.
| METs | Activity | Yes | No |
|---|---|---|---|
| 1 |
| 2.75 1.75 2.75 | 0 0 0 |
| 4 |
| 5.50 8.00 2.70 3.50 8.00 4.50 5.25 6.00 | 0 0 0 0 0 0 0 0 |
| ≥ 10 | 12. Can you participate in strenuous sports like swimming, singles tennis, football, basketball, or skiing? | 7.50 | 0 |
a This is a self-administered questionnaire that assigns a score to each “Yes” or “No” answer; the summed score from the 12 items is the total DASI score, which can be divided by 3.5 to calculate the estimated DASI metabolic equivalents.
Clinical risk assessment tools
A number of risk indices have been developed that incorporate patient- and surgical-related risk factors to predict the risk for perioperative complications. The most commonly known are the Goldman risk index, the Revised Cardiac Risk Index (RCRI), the Myocardial Infarction and Cardiac Arrest (MICA) risk index, and the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator.
Goldman risk index
Developed by Goldman et al. in the 1970s, the Goldman risk index was one of the earliest indices to predict the risk for perioperative mortality and cardiac complications. It incorporates patient demographics, the presence of heart failure, electrocardiographic signs, and type/urgency of operation to predict the risk for perioperative cardiac complications. Detsky et al. updated the Goldman index in 1986, modifying the components of the original index and adding several more variables. Despite these improvements, it was found to have several weaknesses and is no longer a recommended tool to predict risk for perioperative cardiac complications.
Revised cardiac risk index
The RCRI was an update of the original Goldman index, published almost 20 years later. It was derived from a cohort of over 2800 patients undergoing elective noncardiac surgery. It is a simple tool that incorporates six variables into predicting risk: history of ischemic heart disease, heart failure, cerebrovascular disease, diabetes mellitus requiring insulin, renal insufficiency (creatinine concentration >2 mg/dL), and high-risk noncardiac surgery. Because of its ease of use and reliability, it has become one of the most widely used and recommended methods to assess preoperative cardiac risk ( Table 10.5 ).
| Risk Factor | Description | |
|---|---|---|
| Intraperitoneal, intrathoracic, suprainguinal, vascular | |
| Myocardial infarction, positive stress test, current chest pain considered to be because of myocardial ischemia, use of nitrate therapy, pathologic Q waves on ECG | |
| History of heart failure, pulmonary edema, paroxysmal nocturnal dyspnea, rales or S3 gallop, pulmonary vascular redistribution on chest radiograph | |
| History of transient ischemic attack or stroke | |
| Diabetes on insulin | |
| Creatinine >2 mg/dL | |
| Risk of MACE | ||
| Points | Class | Risk |
| 0 | I | 0.4% |
| 1 | II | 0.9% |
| 2 | III | 6.6% |
| ≥3 | IV | 11% |
Gupta myocardial infarction and cardiac arrest risk index
Using data from the American College of Surgeons NSQIP database, Gupta et al. derived the NSQIP MICA risk index. It incorporates five factors: age, functional status, American Society of Anesthesiology (ASA) class, serum creatinine, and type of procedure. It is simpler to use than the Surgical Risk Calculator described in the next section and was shown to have improved discriminatory ability over the RCRI in the derivation cohort.
NSQIP surgical risk calculator
Created by the American College of Surgeons in 2013, the NSQIP Surgical Risk Calculator or model is a comprehensive risk prediction tool derived from data from more than 1 million surgical procedures nationwide. It incorporates more variables than the RCRI, including patient- and surgery-specific indicators, such as the specific type of surgery. The calculator provides an estimated risk for a varied number of perioperative complications, including noncardiovascular complications such as pneumonia and renal failure, and estimated length of stay. It was shown to have excellent predictive ability in the original cohort of patients. Nevertheless, it has not been validated well outside of the original study cohort.
Cardiovascular risk index
Preoperative cardiac evaluation continues to evolve, with new indices being constantly developed to improve the detection of CAD in this setting. Recently, Dakik et al. devised a new risk index called the Cardiovascular Risk Index (CVRI), which incorporates six elements that are easy to obtain: age of at least 75 years, history of heart disease, symptoms of angina/dyspnea, anemia, vascular surgery, and emergency surgery. The CVRI predicts the risk for all-cause mortality, MI, or stroke at 30 days after noncardiac surgery. The authors found that their index performed better than the RCRI (C-statistic: 0.90 vs. 0.78, respectively) and similarly to the ACS NSQIP (C-statistic: 0.90 vs 0.89, respectively). They also externally validated their system in a large population from the NSQIP database.
Current clinical guidelines recommend the use of either the RCRI or the NSQIP models for preoperative risk stratification. , The ideal risk stratification tool should be able to predict perioperative cardiac risk with accuracy and reliability for any given patient population. Nevertheless, no singular risk index is perfect, and it is important to acknowledge the limitations of the risk indices currently available for clinical use. Since initial publication, the original Goldman risk index has been found by multiple studies to have poor discriminatory ability. Although the RCRI has improved accuracy over the original Goldman index, it may have limited ability to discriminate between low- and high-risk patients for specific subgroups, such as those undergoing certain vascular surgeries or organ transplantation. This may be because of the limited number of high-quality studies assessing the applicability of the RCRI across a varied cohort of patients. Another weakness of the RCRI is that it does not incorporate functional status as a variable, which is known to be an important predictor of perioperative outcomes. Moreover, the aforementioned indices were developed more than three decades ago and, since then, many improvements have been made in the medical, surgical, and anesthetic management of patients. The NSQIP is a newer index and comprehensive, but, as a result, it is detailed and more time-consuming than other available risk calculators. In addition, there are a limited number of studies validating its use outside of the cohort included in the derivation of the score. The NSQIP MICA is comparable to the RCRI but incorporates functional status; however, there is limited evidence of its applicability to patients outside of the original derivation cohort. Importantly, despite the valuable information that clinical risk estimation tools provide, the concomitant use of noninvasive testing, and more specifically radionuclide imaging, carries incremental short-term and long-term prognostic value, which will be discussed in the next section.
Noninvasive testing for preoperative risk assessment
Rationale of noninvasive imaging testing
Preoperative cardiac testing has a multifold purpose. First, it allows for the identification of patients with myocardial ischemia who would benefit from either revascularization or medical therapy optimization before undergoing noncardiac surgery. It may also result in adjustments in the perioperative care of these patients based on the test results. Importantly, it also allows clinicians to identify patients who may be at too high a risk of perioperative complications and for whom the risk of planned surgery may be greater than the potential benefit. The results gained from these tests allow clinicians to be better informed when they engage in shared decision making with patients and families. As a result, patients and clinicians may choose to continue with the procedure, cancel the procedure altogether, or choose an alternative procedure with a lower risk profile.
Noninvasive stress testing (pharmacologic or exercise) and imaging techniques are usually the initial approach taken to identify patients with myocardial ischemia, with radionuclide MPI and dobutamine stress echocardiography (DSE) being the most frequently used modalities. There are currently no randomized controlled trials with a head-to-head comparison of MPI with DSE in preoperative cardiac risk evaluation. Nevertheless, there is a wealth of observational data on the utility of each technique. The decision to use MPI or DSE is often based on local practice patterns, resource availability, and clinician expertise and comfort with the technique.
Radionuclide myocardial perfusion imaging
The technical aspects and image acquisition/processing of radionuclide imaging are discussed in detail in Chapter 4 . MPI (single photon emission computed tomography [SPECT] or positron emission tomography [PET]) usually involves visualization and measurement of the uptake of tracer into the heart at rest and during stress (exercise or pharmaceutical). Myocardium that is scarred from prior infarction has lower tracer uptake than the surrounding healthy myocardium and, as a result, both rest and stress images display a similar perfusion “defect,” known as a fixed perfusion defect . In contrast, a reversible perfusion defect, with reduced uptake on stress compared with rest, indicates myocardial ischemia. The LVEF, infarct size, extent of ischemia, and territory at risk can further be quantified using SPECT or PET imaging.
Utility for prediction of perioperative cardiac events
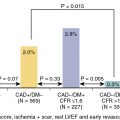
Since the initial study by Boucher et al., the role of radionuclide perfusion imaging in preoperative cardiac risk assessment has been extensively studied. Imaging using SPECT MPI has been shown to have a sensitivity and specificity of approximately 90% and 70%, respectively, for the detection of angiographically significant CAD. Moreover, the presence and size of inducible ischemia demonstrated with MPI has substantial prognostic value. , In a meta-analysis of nine studies with a total of 1179 patients undergoing vascular surgery, Etchells et al. observed that patients with reversible ischemia in more than 20% of the myocardium were at increased risk for perioperative cardiac death and MI. Importantly, a normal MPI study has a very high negative predictive value for MACE, highlighting the value of MPI as a tool in a clinician’s arsenal for preoperative cardiac risk assessment.
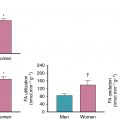
Furthermore, despite the presence of sex-specific differences in diagnostic accuracy for other testing modalities, such as stress ECG, the use of radionuclide imaging has significant clinical value for both male and female patients. This was demonstrated in a study looking at sex differences in outcomes of vascular surgery patients undergoing preoperative dipyridamole thallium imaging, showing that this modality plays a significant role in preoperative risk assessment and long-term prognosis in both male and female patients. Finally, in addition to its clinical and prognostic value, radionuclide imaging has been proven to be a cost-effective resource in preoperative risk assessment when used in the appropriate clinical context.
Long-term risk assessment
Patients with underlying CAD who are at elevated risk for perioperative MACE are also at risk in the long term. Therefore an assessment of long-term risk may play an important role in the decision-making process when planning surgery. The ability of radionuclide MPI to predict long-term events has been demonstrated by multiple studies. Hendel et al. observed that in patients undergoing vascular surgery, the presence of reversible defect on their preoperative dipyridamole 201 thallium MPI was predictive of short-term MACE, whereas fixed defects were predictive of long-term MACE, up to 30 months after surgery. Furthermore, Younis et al. showed that reversible defects were predictors of both short-term and long-term risk. Another study with a longer follow-up period showed that reversible defects were predictors of late cardiac events up to 7 years after endarterectomy, with event rates reaching 50% at 7 years compared with only 2% in patients without demonstrable ischemia. In a meta-analysis of 10 studies with patients undergoing vascular surgery, Shaw et al. found that reversible ischemia predicted perioperative MACE more than fixed defects but that the predictive ability varied with CAD prevalence; on the other hand, a fixed defect was more predictive of long-term MACE. These data show that radionuclide MPI allows for a comprehensive assessment of cardiac risk, which can then be used to better inform the decision-making process on whether or not to proceed with surgery.
Patient-centered approach to preoperative risk assessment
Case vignette 1: Preoperative risk assessment before organ transplanation
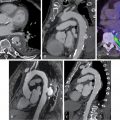
A 55-year-old male with hypertension and cirrhosis secondary to nonalcoholic steatohepatitis complicated by refractory ascites requiring frequent paracentesis underwent evaluation before liver transplantation. He had poor functional capacity at baseline and the initial work-up included a pharmacologic SPECT MPI, which showed a moderate-sized defect of moderate intensity in the anteroseptal, distal anterior, and apical walls and was completely reversible at rest ( Fig. 10.2 ). The patient was then referred for coronary angiography, which revealed significant one-vessel CAD with 80% stenosis in the mid left anterior descending artery (LAD). At that time, PCI was deferred to allow evaluation by hepatology with endoscopy before starting dual antiplatelet therapy and to discuss the need to postpone any surgical intervention. The patient was found to have one large esophageal varix and a multidisciplinary team made the decision to pursue PCI before liver transplant. He then underwent successful PCI of the mid LAD with a drug eluting stent.