Fig. 8.1
A 17-year-old boy with Ewing’s sarcoma of the left pelvis, with extension into the iliac bone, acetabulum, gluteus, and piriformis muscles. As the patient suffered intense pain in the affected areas, the study FDG–PET/CT was done with the patient prone rather than supine
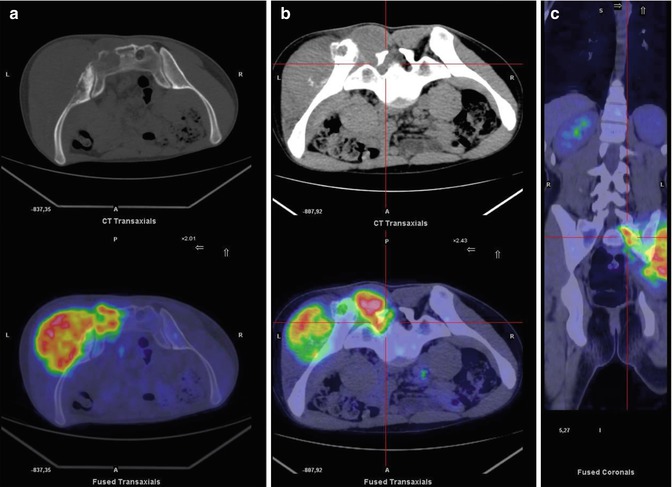
Fig. 8.2
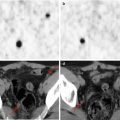
Same patient as in Fig. 8.1. (a) Axial bone window CT and PET/CT fusion images of the prone pelvis show pathological uptake in the left sacrum and iliac bone. On CT, interruption of the posterior cortical bone of the sacrum, a sign of the lesion’s aggressiveness, can be seen (a). Axial soft tissue window setting on CT and axial (b) and coronal PET/CT fusion (c) images of the prone pelvis show pathological uptake in the foramen of the sacral nerve and in the erector muscle of the spine
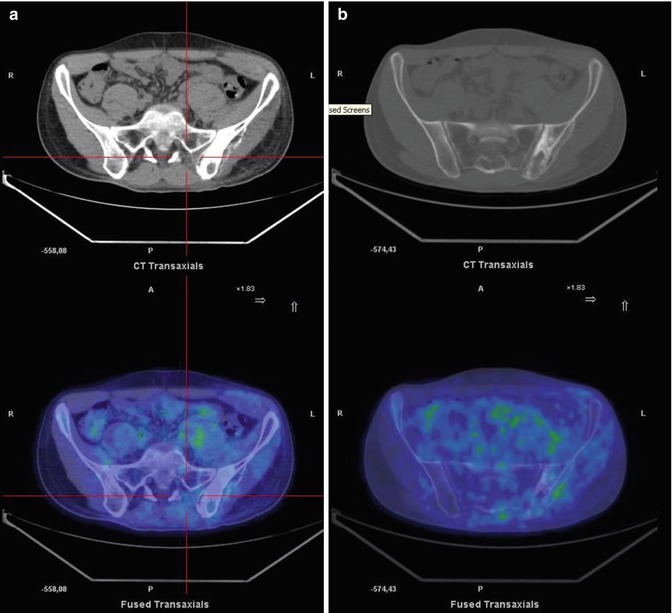
Fig. 8.3
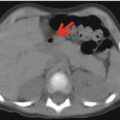
Axial mediastinal (a) and bone (b) windows CT and PET/CT fusion images posttreatment show the complete disappearance of the pathological soft tissue and bone uptake. Reduction of the sarcoma mass reduced the patient’s pain, which allowed image acquisition in the conventional (supine) position
8.3 Lung Metastases in Primary Bone Sarcoma
In up to 20 % of patients with OS or EWS, clinically evident metastatic disease is already present at the time of diagnosis. Among these patients, the lungs will be involved in 92 % [26]. In patients who do not initially present with metastases, the probability of lung metastasis is 28 % after 5 years [27]. Early detection of lung metastases enables disease control by metastasectomy [28–30], which results in a more favorable prognosis (5-year disease-free and overall survival of 10–35 %). By contrast, in patients treated with combination chemotherapy, a complete response is obtained in <10 % of the cases and the prognosis is poor [31].
High-resolution CT, due to its high sensitivity, remains the gold standard in detecting lung metastases, although difficulties are encountered in defining the true nature of lung nodules in these patients. FDG–PET/CT has good specificity in the assessment of suspicious nodules and better diagnostic performance than other imaging methods when the diameter of the lung lesion is >6 mm [20] because of the limited resolution of PET or CT alone and the artifacts that arise in these studies from partial volume effects and respiratory movement. Instead, the higher specificity of 18F-FDG–PET/CT reinforces the complementary roles of these other modalities in the assessment of lung lesions (Figs. 8.4, 8.5, and 8.6) such that indiscriminate use of surgical biopsies and nodulectomies is often avoided [20].
Lung nodules, frequently small in size and multiple, must be evaluated with a semiquantitative or qualitative approach. The classical SUVmax cutoff value (2.5) used to discriminate between benign and malignant lung nodules is not suitable in pediatric oncology patients because their lung lesions differ from those of adults with respect to glucose metabolism. Furthermore, in the clinical history, several conditions must be considered, such as recent cough, fever, and infections, all of which can generate benign pulmonary nodules with high glucose metabolism that are mistakenly interpreted as tumors. Cistaro et al. attempted to establish an SUVmax cutoff value that allowed the correct diagnosis of pulmonary nodules in pediatric patients with bone sarcomas. Whereas for lesions <5 mm significant SUVmax (and the SUV ratio) values could not be defined, an SUVmax threshold >1.09 was found to be consistent with malignancy for nodules >6 mm in diameter. The authors concluded that knowledge of the patient’s clinical history combined with the use of a semiquantitative approach may diminish the number of false-positive malignant nodules. Overall, 18F-FDG–PET had an accuracy of 88.9 %, a sensitivity of 90.3 %, a specificity of 87.5 %, and a PPV and NPV of 90.3 and 87.5 %, respectively [30].
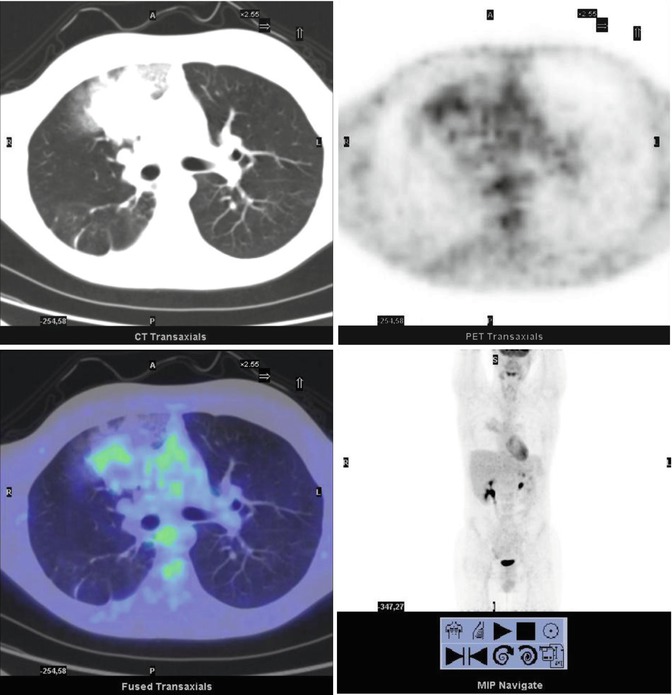
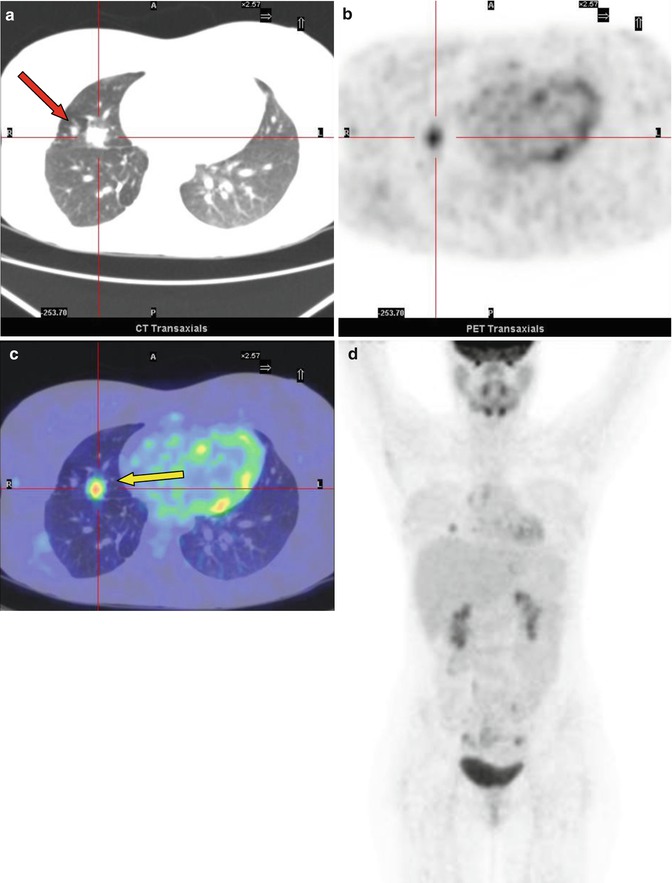
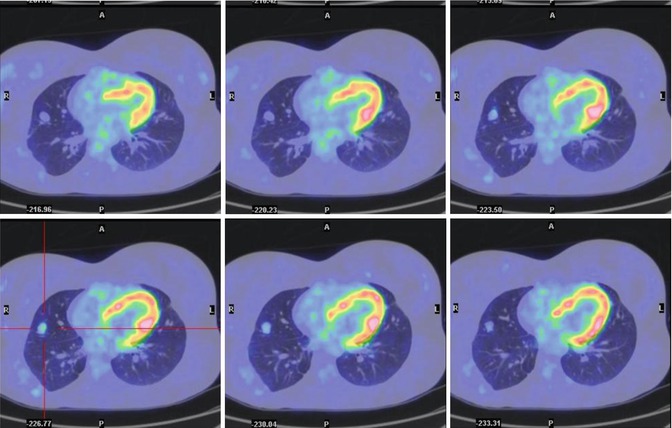

Fig. 8.4
A 14-year-old boy with Fraumeni syndrome who was treated for bilateral retinoblastoma and osteoblastic osteosarcoma of the right femur. The PET restaging study showed inhomogeneous uptake in the right upper lobe of the lung (SUVmax 2.4). The patient underwent resection of the lesion, subsequently diagnosed as a metastasis without necrosis

Fig. 8.5
A 21-year-old female treated 7 years earlier for osteosarcoma of the humerus, including seven pulmonary metastasectomies. (a–d) Axial lung windows on CT and PET/CT fusion images show focal FDG uptake corresponding to a nodule on the right upper lobe of the lung (SUVmax = 5:7) (yellow arrow in c). The nodule was interpreted as of inflammatory origin and the patient was treated with antibiotics. A chest CT 15 days later confirmed the inflammatory nature of the lesion. On CT, another small nodule (red arrow in a) initially detected 2 years earlier is seen

Fig. 8.6
The same patient 6 months later. Axial PET/CT fusion images show a mild increase in the dimensions and degree of 18F-FDG uptake by the small nodule (SUVmax = 2.3). The patient underwent lung surgery. The final diagnosis was metastasis with poor necrosis
8.4 Bone Metastases
No consistent data on the risk and sites of extrapulmonary metastasis from bone sarcomas are available. However, the bones seem to be the more frequent site of extrapulmonary metastases, as bone metastases develop in 33 % of patients (Fig. 8.7) [31]. Since the bones are not connected to the lymphatic system, tumor dissemination is almost exclusively through the blood.
FDG–PET/CT directly identifies bone lesions on the basis of their glucose metabolism and is thus better able to detect the osseous metastases of EWS than 99mTc bone scintigraphy [32]. Nonetheless, despite its lower resolution, bone scintigraphy has a high detection rate for skeletal OS metastases. This most likely reflects the intense osteoid production and osteoblastic activity of OS. EWS tends to infiltrate the bone marrow rather than mineralized bone such that osteodestruction is dominated by osteoclastic activity [33, 34].
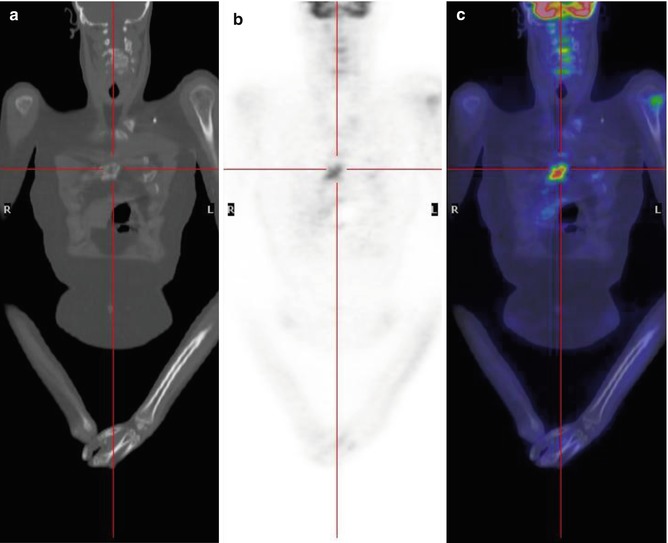

Fig. 8.7
A 17-year-old boy treated for sarcoma. Coronal CT (a), PET (b), and PET/CT fusion (c) images show mild FDG uptake in the left humeral head and another, more intense accumulation in the sternum
8.5 Other Sites of Metastases
Extrapulmonary and extraosseous lesions are extremely rare. According to the limited data on lymph node involvement, metastatic lymph nodes are seen in 10 % of the OS cases at autopsy [35]. FDG–PET/CT effectively detects lymph node metastases (Fig. 8.8) as well as metastases in other, more unusual sites (<3 % of patients with metastatic disease), e.g., brain, liver, muscles, abdomen, and scalp [20, 26]. A large retrospective study by the Cooperative German–Austrian–Swiss Osteosarcoma Study Group yielded detailed data on the distribution of OS metastases; in 12.4 % of the 1702 OS patients, metastases were present at the time of diagnosis. The lungs were involved in 86.7 % patients, distant bones in 21.2 %, and other sites in 0.09 % (lymph nodes in 15 patients, other soft tissues in 3, skin, brain, and liver in 1 patient each) [36]. The European Intergroup Cooperative Ewing’s Sarcoma Study Group collected data from 975 EWS patients. In 179 (18.4 %), metastases were detected at initial presentation. In 79 of those patients, the lungs were the only site involved by metastasis, while in 92 patients, bone with or without lung involvement was present. Eight patients had metastases at other sites, and in one patient, the metastatic site was not specified [17]. In patients with metastatic disease, FDG–PET/CT can identify extrapulmonary lesions with a higher sensitivity, specificity, and accuracy than provided by conventional imaging (83.3 vs. 77.8%, 98.1 vs. 96.7 %, 96.9 vs. 95.2 %, respectively) [20].
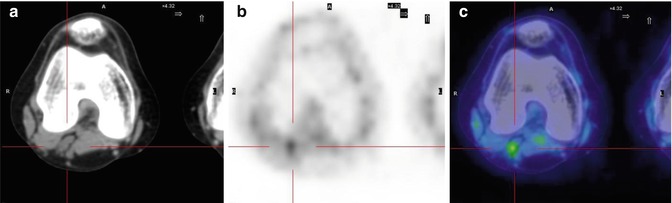

Fig. 8.8
A 15-year-old girl treated for Ewing’s sarcoma of the right fibula. Axial CT (a), PET (b), and PET/CT (c) fusion images show a small focal area of uptake in the left popliteal fossa, indicating recurrence of the disease in the lymph nodes
8.6 Local Recurrence
Since pediatric patients with bone sarcoma usually undergo surgery in combination with robust chemotherapy and radiotherapy protocols, they are likely to develop tissue scarring, fibrosis, and inflammation that on imaging can simulate recurrent disease. Assessment of local recurrence by means of morphological imaging can be difficult because of tissue changes following previous treatment. In addition, metallic prostheses can cause significant artifacts on MRI. In these settings, FDG–PET/CT may be diagnostically useful as it is able to differentiate viable tumor from fibrosis and inflammatory tissue from malignancy (Figs. 8.9 and 8.10) [21]. The sensitivity, specificity, accuracy, NPV, and PPV of FDG–PET/CT in discriminating local recurrence from bone tumors were reported to be 100, 92, 95, 100, and 88 %, respectively [37].
Patients who suffer a relapse of primary bone cancer have a poor prognosis [38]. In a retrospective analysis of 114 OS patients, McTiernan and colleagues reported a 5-year estimate of post-relapse survival (PRS) of 19.2 ± 7.7 %. A much worse outcome (5-year PRS = 13.3 ± 8.8 %) was documented for patients with simultaneous local and distant recurrence than for patients with local recurrence alone (27.3 ± 11.6 %), but the difference was not statistically significant [37].
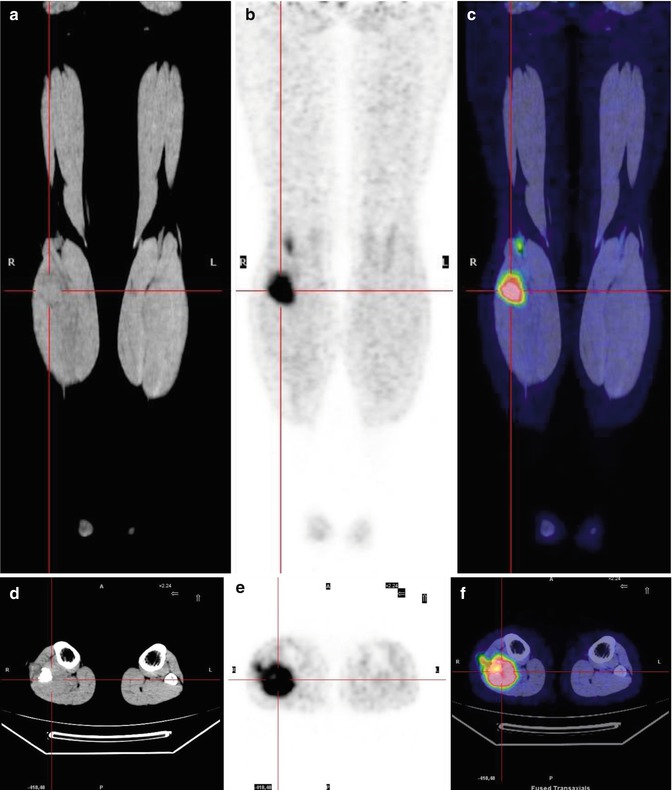
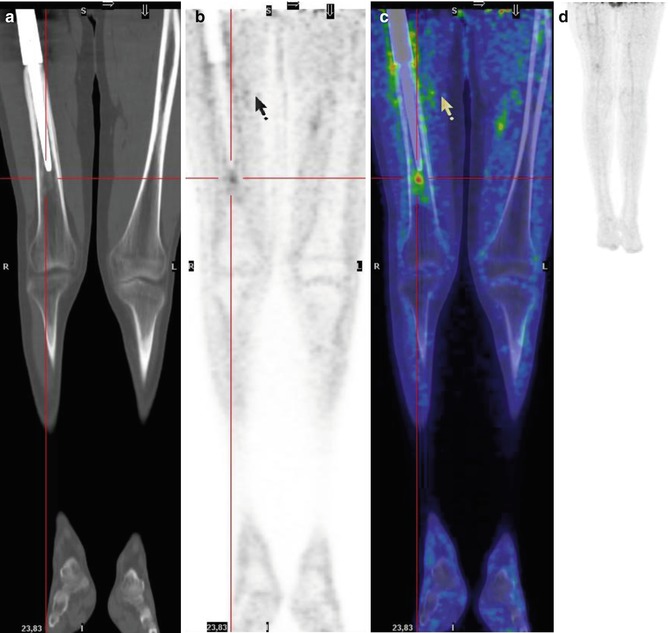

Fig. 8.9
A 15-year-old girl previously treated for Ewing’s sarcoma of the right fibula. The coronal (a–c) and axial (d–f) CT (a, d), PET (b, e), and PET/CT fusion (d, f) images show a right soft tissue recurrence between the popliteal muscles, involving the proximal fibula

Fig. 8.10
A 15-year-old boy treated for osteosarcoma of the left femur. Coronal CT (a), PET (b), and PET/CT fusion (c) images show focal uptake at the apex of the prosthesis due to static–dynamic alterations during walking and standing. (d) MIP shows the different lengths of the legs, with the left being shorter than the right
8.7 Therapy Monitoring
In OS, a further application of FDG–PET/CT is to monitor the response of the primary tumor or the metastases to chemotherapy, including to new therapeutic protocols (Figs. 8.11, 8.12, and 8.13). While the therapeutic response of bone tumor lesions is usually assessed on the basis of morphological changes on CT and MRI, in bone tumors, a change in the size of the lesion is not always a reliable parameter. By contrast, in the follow-up of neoplastic lesions, FDG uptake is significantly related to tumor or metastatic response and, in this context, is superior to morphological assessment. In addition, identification of the most active site of the mass can also guide the clinician in choosing the best biopsy site as well as in devising a tailored radiotherapy plan [32, 39–41]. Some authors have suggested the use of a post-therapy SUVmax cutoff (2.5) response or an SUV2: SUV1 ratio of 0.5 to discriminate between good and poor responses [42]. Differences in the therapeutic response criteria used in the evaluation of OS and EWS have also been noted. Using histological regression as the reference, Denecke et al. studied 27 patients with EWS and OS who were evaluated by FDG–PET/CT and MRI before and after neoadjuvant chemotherapy prior to tumor resection. In the subgroup of OS patients, FDG–PET/CT was superior to MRI in the noninvasive response assessment, whereas in EWS patients, neither FDG–PET nor MRI criteria enabled a reliable response assessment [41]. However, these conclusions were based on small series and further investigations are warranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree