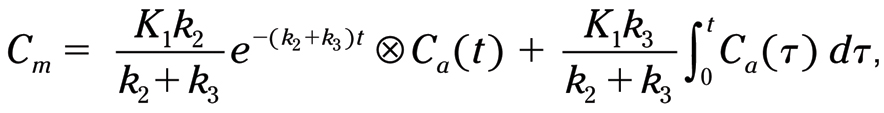- •
Accurate quantitative MBF quantification requires an understanding of the technical capabilities of the instrumentation and quantitative software used to make the measurements.
- •
Quality control of MBF studies includes careful assessments of the timing and quality of the injected radiotracer bolus, proper placement of the myocardial blood pool ROI, correction of patient motion during the dynamic scan, and inspection of the overall count density and detector saturation during the dynamic image data set.
- •
The administered dose must be adjusted to match the specifications of the PET and SPECT camera to obtain quality images and avoid detector saturation during the blood pool phase.
- •
Multiple kinetic models are available and each have their own strengths and weaknesses. Selection of the most appropriate model also depends on the instrumentation and software available to make the blood flow measurements.
- •
The use of conventional SPECT instrumentation for blood flow measurements is challenged by the need to obtain rapid tomographic images, especially at the beginning of the dynamic acquisition, and maintain linearity throughout a wide range of count rates. In addition, the limited extraction of currently available SPECT tracers also contributes to the limitations of SPECT.
Introduction
Absolute myocardial blood flow (MBF) assessments for nuclear cardiology add unique information that is difficult, if not impossible, to acquire using other modalities. Specifically, the assessment of absolute MBF with cardiac positron emission tomography (PET) improves the determination of normalcy, , detection of multivessel disease, and assessment of patient prognosis. An absolute MBF assessment has recently been demonstrated in a large study of 12,594 patients to be effective in assessing which patients may benefit from revascularization. An MBF assessment using single photon emission computed tomography (SPECT) has also demonstrated potential for absolute MBF assessment. Nevertheless, these benefits can only be obtained if the quantitative values are accurate.
The assessment of MBF uses measurements of the concentration of radiotracer in the blood as a function of time and the uptake of that tracer and also uses a model that describes the kinetics of the tracer. This measurement uses a set of dynamic tomographic images beginning at the time of tracer infusion and following the transport of the tracer into the myocytes. Because of the rapidly changing tracer concentration during the initial infusion, these dynamic tomographic images must be acquired in short intervals. In addition, these dynamic images must be quantitatively accurate. This can be challenging owing to differences in scanner sensitivity and the wide range of count rates that may be present at the beginning, middle, and end of the acquisition. For many PET blood flow protocols, the count rate can be 10 times higher during the initial bolus of activity than the count rate during the perfusion scan. Also, in the case of 82 Rubidium ( 82 Rb), the activity will decay to near background levels during the course of the study. This complex, kinematically dynamic and quantitative study must be accomplished without adding to the radiation dose or compromising the quality of the clinical myocardial perfusion study. ,
The choice of the radiotracer used to measure blood flow, in principle, should not have an impact on coronary blood flow; thus, blood flow measurements should be independent of the radiotracer used. In practice, the radiotracer’s first-pass extraction fraction plays a vital role in determining the accuracy and precision of blood flow measurements. Therefore, protocols and models for measuring MBF are specific to the radiotracer used. Radiotracers with higher first-pass extraction fractions tend to create a greater contrast between normal and abnormal regions. Quality control and data processing steps depend heavily on the choice of radiotracer.
MBF assessment with SPECT is even more challenging than with PET. Conventional Anger SPECT systems cannot acquire the rapid dynamic studies necessary for quantitation of the arterial input function. There have been some studies that have attempted to use a fast rotation scanning protocol to acquire the dynamic data sets , ; however, most conventional SPECT camera gantries are unable to scan at the necessary rotation rates. An alternate approach is to use either a set of small cadmium-zinc-telluride (CZT) scanners capable of a fast sweeping acquisition , or a multipinhole dynamic acquisition that does not require rotation.
Ultimately, the assessment of MBF greatly increases the diagnostic information available to the cardiologist. As discussed throughout this textbook in patient-centered applications of radionuclide imaging, this new information complements the visual assessment of the study; however, the utility of an absolute blood flow assessment requires an understanding of the entire acquisition, processing, and quality control procedures to ensure that the measurements are accurate and reliable.
Myocardial blood flow measurements with PET and SPECT
PET tracers for measuring myocardial blood flow
In principle, with an accurate model of a PET tracer’s extraction and retention properties and a high-quality dynamic acquisition, all PET tracers would produce equivalent results. Nevertheless, variations in each tracer uptake and retention introduces both systematic and random errors into the measurement of MBF.
One of the most important considerations for the accuracy of absolute blood flow determination is the linearity of tracer extraction relative to MBF. The better the extraction of the tracer at high flow rates, the more sensitive the tracer is at detecting small changes in absolute blood flow. Fig. 3.1 illustrates the relationship between the extraction rate ( K 1 ) and MBF for several SPECT and PET tracers.
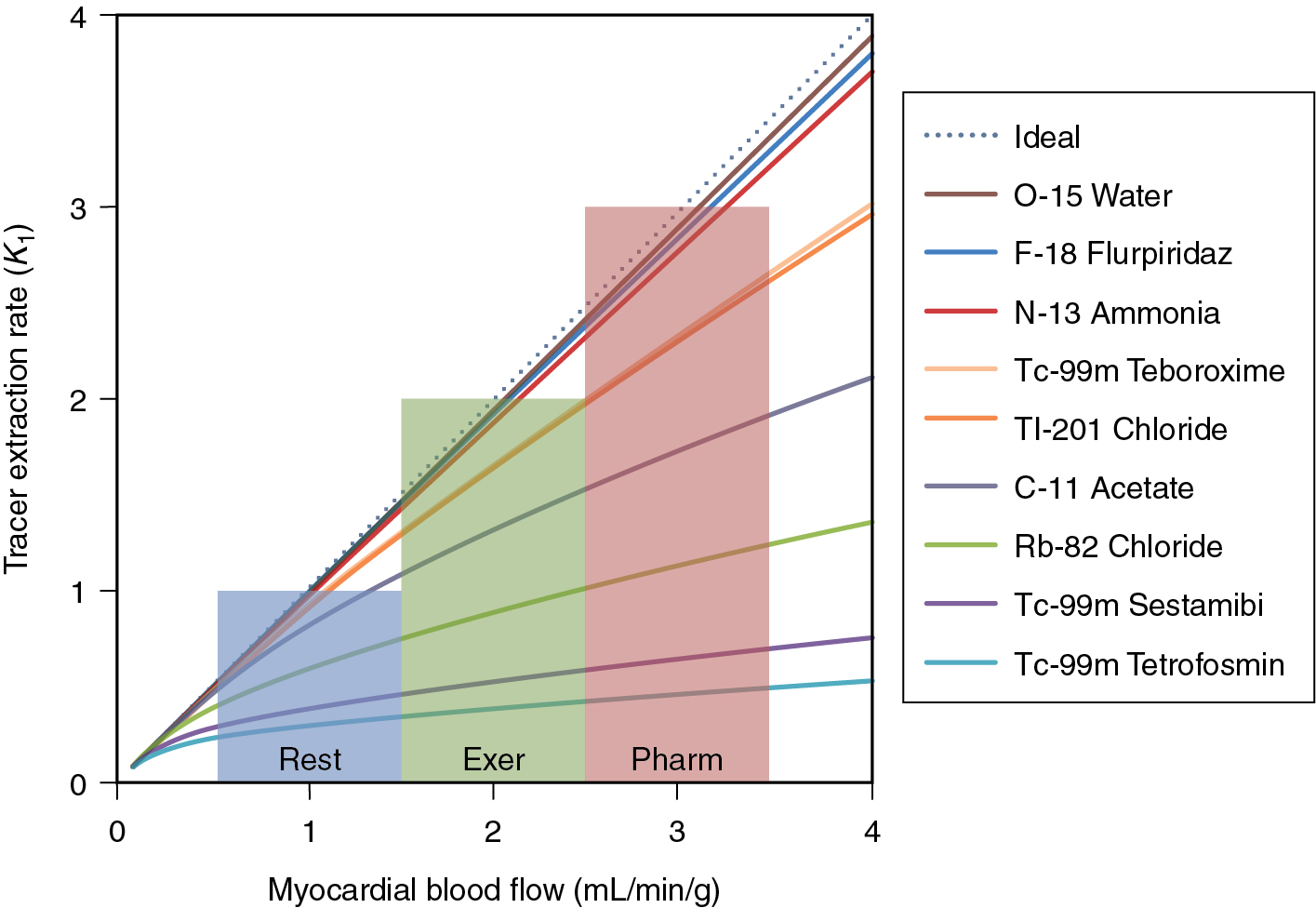
15 O-water
The gold standard for the estimation of MBF is oxygen-15-labeled water ( 15 O-water). This tracer has particularly favorable characteristics by freely diffusing across the cell boundary. This property gives 15 O-water a linear 1-1 relationship between K 1 and blood flow. A major challenge with 15 O-water is the very short half-life of 15 O-water (122 seconds). This makes delivery of the radiotracer very difficult. In addition, 15 O-water is not retained in the cell and is best imaged parametrically, making traditional assessment of wall-motion thickening and visual interpretation challenging, if not impossible.
82 Rubidium
82 Rb is the most commonly used PET perfusion agent in the world. It is most commonly delivered using an infusion system as rubidium chloride solution. As a potassium analog, rubidium is absorbed into myocytes via the sodium potassium pump. Once inside the cell, rubidium is not bound and will slowly wash out (k 2 < 0.14/minutes at rest). 82 Rb properties are similar to 201 thallium.
Because of the efficient uptake and slow wash-out rate, 82 Rb can be modeled well with a net retention, single-tissue compartment model (1TCM) or a two-tissue compartment model (2TCM). For the net retention model, a short (<3 minutes) dynamic acquisition is necessary to avoid errors from tracer washout. For longer acquisitions, either the 1TCM or 2TCM is recommended. ,
13 N-ammonia
13 N-ammonia is a cyclotron-produced tracer with a 9.93-minute half-life. This radiotracer has excellent first-pass extraction and a relatively long half-life and produces high-quality perfusion and functional images. Quantitative measurements of MBF are complicated by the fact that 13 N-ammonia is only partially retained in the cell. Upon first extraction from the blood pool, a portion of the ammonia is converted to glutamine and retained in the cell. A smaller fraction is then converted into a 13 N metabolite and washes out into the blood pool. This metabolite cannot be re-extracted from the blood pool and can artifactually increase the estimate of the arterial input function. For an accurate measurement of MBF with ammonia, a 2TCM that includes corrections for the metabolite must be used. ,
18 F flurpiridaz
A new 18 F-labeled tracer, 18 F flurpiridaz, is currently under Phase III investigation as a myocardial perfusion agent. Preliminary studies of this agent demonstrate a high signal to background, high extraction fraction, and excellent resolution properties. , In addition, this agent has the advantage of unit dose delivery, thereby opening opportunities for cardiac PET without the challenge of owning a generator or cyclotron. More recently, quantitative blood flow modeling has indicated that 18 F flurpiridaz has superior flow characteristics than 82 Rb comparable flow characteristics to 13 N-ammonia with a high extraction fraction (0.94) that is constant up to flows of 5.06 mL/g/minute.
Kinetic modeling
Calculating MBF from measurements of radiotracer uptake and blood pool concentration requires a model for tracer transport into myocytes. The most common model for tracer transport is a multicompartment model. Each compartment of the model represents a location the tracer can be in during the acquisition. In the simplest model, the tracer would begin in the blood (compartment one) and then be transported into the cell (compartment two). More complicated models would include parameters for washout from the myocyte, compartments for the interstitial layer, metabolism of the tracer, and different subcomponents within the cell ( Fig. 3.2 ).
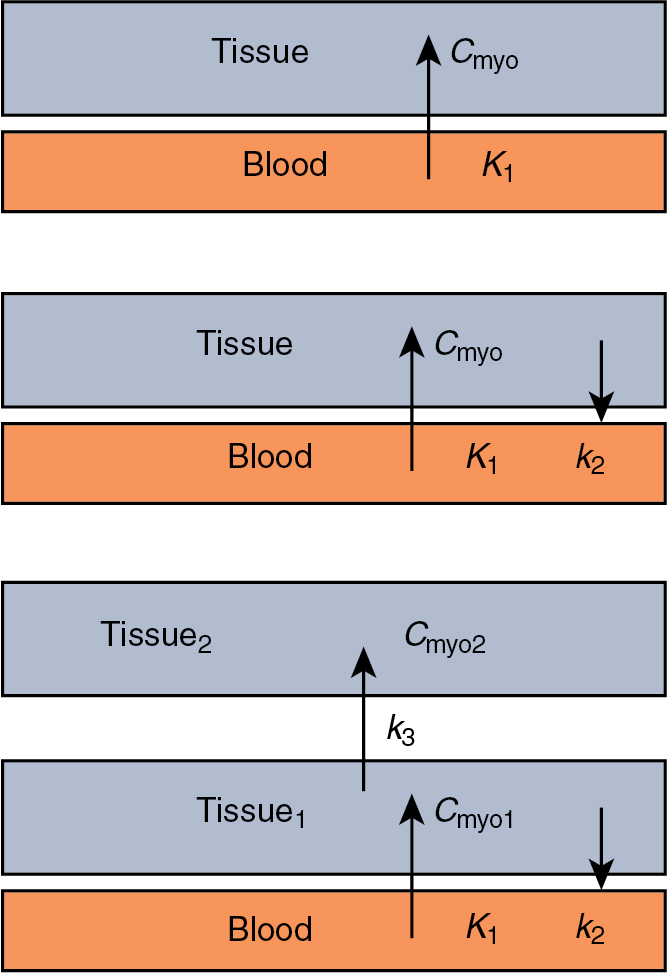
The choice of a compartmental model for calculating MBF is based on the underlying kinetics of the tracer and the parameters of the acquisition. In addition, the choice of a compartmental model should take into consideration common imaging artifacts, such as image noise, patient motion, and noncardiac uptake.
Most myocardial perfusion tracers are modeled accurately using a 2TCM, where one compartment represents the blood and the two tissue compartments could represent the cell, an interstitial layer, or subcomponent in the cell. The general form of a 2TCM is :
where C m is the myocardial uptake, K 1 is the uptake into the first tissue compartment, k 2 is the washout from the first compartment, k 3 is the uptake into the second tissue compartment, t is the timepoint the uptake is measured, and C a (t) is the arterial concentration at timepoint.
To calculate the blood flow from the compartmental model, a model for the tracer extraction is needed. The tracer extraction from the blood is often modeled using the Renkin-Crone formula , :
K1=MBF⋅(1−a×exp(−bMBF)),
where MBF is the myocardial blood flow and a and b are fit parameters. A list of common a and b parameters is given in Table 3.1 . , , ,
| Rb-82 | N-13 Ammonia | F-18 Flurpiridaz | Tc-99m Sestamibi | Tc-99m Tetrofosmin | Tl-201 | |
|---|---|---|---|---|---|---|
| a | 0.77 | 0.096 | 0.07 | 0.27 | 0.21 | 1.4 |
| b | 0.63 | 1.08 | 1.6 | 0.87 | 0.93 | 0.37 |
Early studies of the 2TCM for 13 N-ammonia demonstrated a high degree of accuracy compared with microspheres. Choi et al. examined the quantitative MBF of 13 N-ammonia using four different quantitative models. In that study, the 2TCM was demonstrated to have low dispersion (0.15) and correlated well with microspheres’ results. The authors also noted the importance of correction of washed-out metabolite. In a separate study comparing blood flow models using 13 N-ammonia, it was demonstrated that the 1TCM did not accurately represent MBF as well as the 2TCM did.
82 Rb is a potassium analog and thus is not metabolized by the myocytes. Because of this, the flow model can be simplified using a 1TCM. Lortie et. al. presented a simplified 1TCM for calculating the MBF using 82 Rb. This model used a nonlinear fit to a series of dynamic measurements of tracer uptake versus arterial blood pool to estimate the K 1 (uptake), k 2 (washout), and partial volume corrections (f).
The 1TCM can be derived from Equation 1 by setting k 3 = 0 (10):
1TCM has been shown to accurately represent the estimate of MBF with 82 Rb and is highly repeatable. ,
This model can be further simplified by setting k 2 = 0. This model is referred to as the net retention model . Comparison of the net retention model with the 1TCM demonstrates a high degree of correlation between blood flow calculations in the first 2 minutes of a study. After the first 2 minutes, the influence of the washout reduces the accuracy of this model. One of the advantages of this simple model is that it can be performed on nonlist mode, frame mode PET systems. Another advantage of this technique is that the myocardial uptake is calculated from a single late frame, making it less susceptible to motion artifacts. One of the limitations of this technique is that partial-volume correction cannot be determined from the data and must be supplied as a parameter.
In a large study comparing MBF calculation techniques with 82 Rb, net retention, 1TCM, and 2TCM models produced similar results. This result confirms the overall robustness of these methods. In addition, software packages using the same compartmental modeling algorithm produced interchangeable results. This further validates the consistency of these software packages, despite varying implementation strategies. The key limitation of this study was that no gold standard was available and therefore the overall accuracy of particular techniques could not be assessed relative to true MBF.
PET imaging protocols
The assessment of MBF with PET is made possible because of two characteristics of PET imaging: (1) All cardiac PET studies are attenuation- and scatter-corrected and (2) PET studies are acquired using a full 360 degrees of data simultaneously, allowing for the creation of rapid, high-quality, dynamic tomographic images.
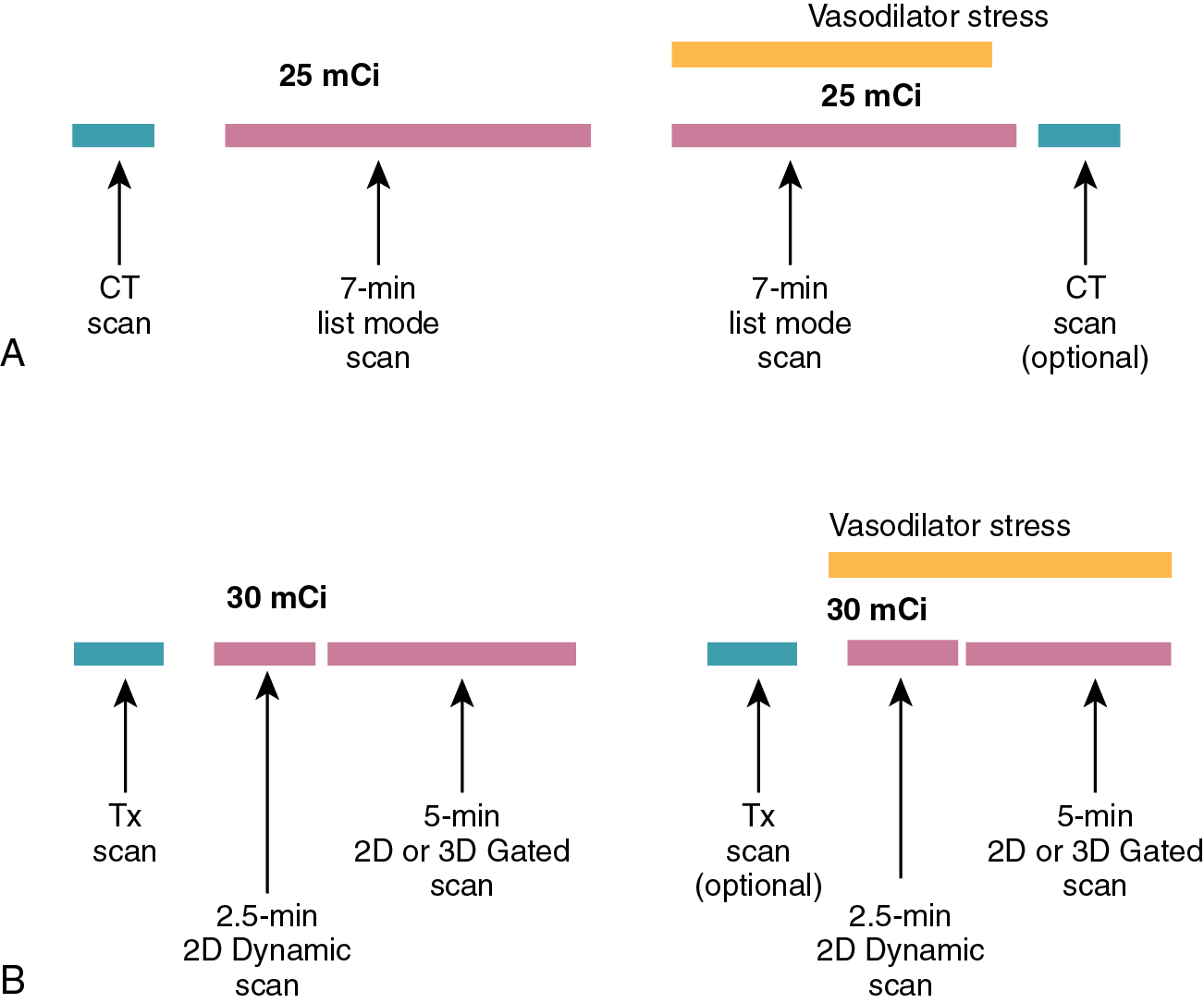
The dynamic acquisition used for the assessment of MBF uses a series of short tomographic images over the duration of the study to measure the transport of the tracer from the blood pool to the myocardium. The most common technique for acquiring the dynamic data is to use a list mode acquisition. These acquisitions record each true coincidence event in a file so that multiple virtual “acquisitions” can be created after the acquisition of the images. This type of acquisition allows for rebinning of the gated, static, and dynamic myocardial perfusion data sets from a single acquisition data set ( Fig. 3.3 a).