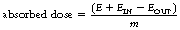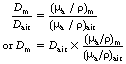Chapter 27 Principles of radiation dosimetry
Chapter contents
27.1 Aim
The aim of this chapter is to introduce the reader to the concepts of exposure, absorbed dose and dose equivalent. It then goes on to consider methods of absolute measurement of radiation dose and different relative methods of dose measurement.
27.2 Introduction
As we saw in Chapter 1 of this book, we live in an environment where we are continuously subjected to ionizing radiation from natural causes such as cosmic rays and naturally occurring radionuclides. In fact, about 90% of the average UK radiation dose comes from natural sources. In addition, there are artificial contributions to the radiation dose because of fallout from weapons testing, leakage from nuclear power plants, manufacture of radionuclides and medical exposure to radiation. All ionizing radiations, whether natural or artificial, constitute a hazard. It is assumed that the greater the radiation dose to which the population is exposed, the greater the hazard. The accurate measurement of radiation dose received by the population is therefore important in trying to quantify the hazard. As can be seen from Figure 1.4, medical radiation constitutes the largest single contribution of the artificial radiation exposure to the population in the UK and so it is important to minimize this radiation dose and hence the total population dose. However, the hazards associated with medical irradiation must be considered against the benefits of diagnosis and treatment. This risk–benefit concept will be discussed in Chapter 44.
27.3 Units of exposure and dose
• the formation of electrical charges in the air by ionization
• the absorption of energy by the air as the electrical charges are slowed down by collision with the air molecules (thus producing further ionization)
• the consequent production of heat energy because of the transfer of energy to the air molecules.
27.3.2 Absorbed dose and kerma
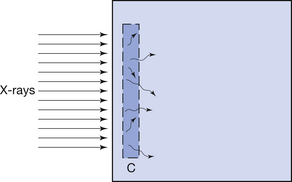
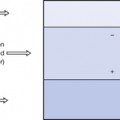
This effect is illustrated in Figure 27.1 (See page 199). Here an incoming X-ray beam of high energy interacts with a volume element V within the medium. Because of the high energy of the beam, the electrons produced by Compton scatter are scattered in a forward direction, so much of their energy is absorbed outside the volume V. There will also be secondary ionizations resulting in the production of delta rays, but for simplicity these are not shown in the figure. In general, if the secondary electrons produced within the volume deposit a total energy E within the medium, and EIN and EOUT are the total energies of the electrons entering and escaping from the volume, then the absorbed dose in grays is given by:
Electronic equilibrium is said to occur if EIN=EOUT, since there is no net loss or gain of the electrons over the small volume being considered. If EIN=EOUT is a constant value not equal to zero, there is said to be quasielectronic equilibrium. If the intensity of the radiation is varied, the net loss or gain of electrons will vary in proportion. An example of electronic equilibrium occurs in the free-air ionization chamber, which will be discussed later in Section 27.5.2.
The absorbed dose expresses the quantity of energy absorbed in the medium due to a beam of ionizing radiation passing through it. As stated at the beginning of this section, the site of the attenuating events (e.g. photoelectric absorption) may be at some distance from the absorption process because of the distance travelled by the ejected electrons before coming to rest. The quantity which measures the amount of attenuation in a small volume is called the kerma (see Sect. 27.3.1).
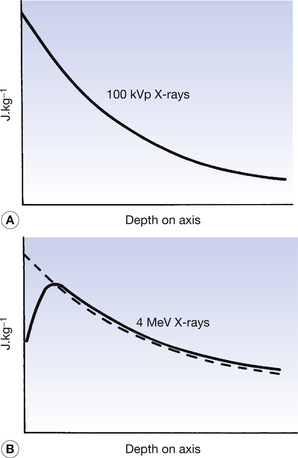
The absorbed dose and kerma along the axis of a beam of X radiation are shown in Figure 27.2. Figure 27.2A shows the case where an X-ray beam generated at 100 kVp is incident upon soft tissue: this type of situation might occur in diagnostic radiography. The electrons released in the primary and secondary ionizations are of relatively low energy and so are absorbed close to the site of the initial attenuating interactions. The kerma and absorbed dose at any particular point along the beam axis are essentially the same and the curves are coincident in the figure. This is not the case if the X-ray beam has high photon energy, since electrons produced by the initial ionization have considerable energy and so deposit their energy some distance from the point of the original attenuation process. As can be seen in Figure 27.2B, the kerma and the absorbed dose due to 4 MeV X-rays interacting with tissue are not the same. It may be easier to understand these curves if it is remembered that:
• the kerma is a measure of the attenuation – the number of photoelectric and Compton events
• the absorbed dose is a measure of the energy deposited in the medium by the primary and secondary electrons being brought to rest.
27.3.3 Effects of different media
Instruments that are used to measure absorbed dose or absorbed dose rate are called dosimeters and dose-rate meters respectively. Some of these instruments are described in more detail in later sections of this chapter (see Sect. 27.5 onwards). It is common practice to calibrate these meters to read the absorbed dose or dose rate in air through which the X- or gamma-rays are passing. Such a dosimeter may read 0.5 mGy as the total absorbed dose in air at a point within an X-ray beam. It must not be inferred, however, that this is the absorbed dose that would bereceived by any other medium if placed in the same position. For two media to receive the same absorbed dose, each must absorb the same energy from the beam per unit mass (remember, 1 Gy=1 J.kg−1). This is the same as saying that the mass absorption coefficient of the two media must be equal. Thus, if Dair is the absorbed dose in air and Dm the absorbed dose in a medium when both are irradiated with the same beam of X-rays, it follows that if the mass absorption coefficients are not equal, this equation may by drawn up:
If the mass absorption coefficient of air and the given medium are known at the energy of the X-ray quanta, the absorbed dose in the medium may be calculated using Equation 27.2. In practice, this allows us to measure the absorbed dose in air at a certain point and then calculate the absorbed dose in the patient at the same point without subjecting the patient to a great degree of discomfort.
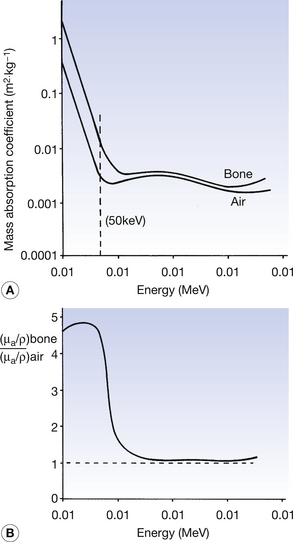
The mass absorption coefficients of both air and bone vary with photon energy. These variations of the two coefficients are shown in Figure 27.3A and the variations in the ratio of the two coefficients are shown in Figure 27 3B. As can be seen from the graphs, at low photon energies (50 keV is shown with the broken line in Figure 27.3A), the mass absorption coefficient of bone is considerably higher than that of air. This is because at low energies the photoelectric effect predominates (τ/ρ ∝ Z3/E3) and the atomic number of bone (Z=14) is approximately double that of air (Z=7.64). For this reason and because of the large difference in density, we get a high level of contrast between bone and air on a radiograph (this can be seen on the chest radiograph or on radiographs of the paranasal sinuses). At an energy of about 1 MeV, however, the two graphs are very close and the ratio of the two coefficients approaches 1. This is because of the dominance of Compton scatter in this region (σ/ρ ∝ electron density and the electron density for bone and air is approximately the same). This means that there would be a low level of contrast between the two if they were radiographed using 1-MeV photons. At above about 10 MeV the curves again diverge owing to the greater amount of pair production in bone compared to air ((π/ρ ∝ Z). Thus, it is clear that an instrument calibrated to read absorbed dose in air must be used with caution when calculating the absorbed dose in another medium as the relationships between the absorption coefficients vary with the photon energies. This is particularly the case in the diagnostic range of energies where absorption is principally by the photoelectric effect, which is very sensitive to both atomic number and photon energy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree