, Valery Kornienko2 and Igor Pronin2
(1)
N.N. Blockhin Russian Cancer Research Center, Moscow, Russia
(2)
N.N. Burdenko National Scientific and Practical Center for Neurosurgery, Moscow, Russia
Prostate cancer (PrC) is the most common solid tumor in men and the second by mortality rate in male population (Jeman et al. 2007). According to Jemal et al. (2011), the estimated number of PrC cases in 2016 in the USA will reach nearly 181,000 people, which makes up 21% of all cancers in men, and 126,000 will presumably die from this disease (8%). In Russia, almost 27,000 males develop PrC annually. According to Davydov and Axel (2014a, b), in the structure of morbidity of malignant neoplasms, PrC amounts to 12.1% and is the leader by an increase in its incidence rate in the male population (35.8%).
Although the incidence and survival rates vary widely in different EU countries, mortality rates do not differ significantly. PrC mortality tends to decrease, both in the USA and in the EU. The survival rate has increased, probably due to more active implementation of early diagnostic procedures and screening programs. Thus, the 10-year and 15-year survival rate has already reached 93% and 77%, respectively. Studies showed that men diagnosed with PrC in the early stages had a minimal risk of death from cancer during 20 years after the diagnosis. PrC in elderly men is a greater problem in the developed countries, where the percentage of relatively older men is higher. Thus, according to Steliarova-Foucher and Parkin (2011), PrC accounts to about 15% of cancers in men in developed countries, whereas it is only 4% in developing countries. It should be noted that the incidence of PrC varies considerably depending on the region. For example, according to the data published by the Swedish National Board of Health and Welfare, Stockholm, in this country, characterized by a high life expectancy and a relatively low mortality from diseases associated with smoking, PrC accounted for 37% of all new cancer cases in 2004.
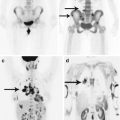
PrC most often metastasizes to the bones, liver, lungs, and lymph nodes and rarely to the brain. According to different authors, bone tissue metastases in PrC amount from 54 to 85%. Thus, according to Ganova et al. (2014), who studied PrC metastases in young and middle-aged men, more than 80% of the lesions are located in the bones of the pelvis and the lumbar spine. The authors noted that 73% of patients were aged 54–59 years.
PrC metastases to the bones are mostly multiple, often of an osteoblastic type, though there are also lytic lesions. Note that bone involvement occurs in 95% of PrC patients with brain metastases. Concomitant involvement of the bones and lungs accounts for 31% of cases, while of the bones, lungs, and liver accounts for 19%.
Metastases to the brain in the presence of concomitant visceral metastases indicate an active process in the internal organs. The combination of metastatic brain lesions with other organ involvement is typical of PrC. Prostate cancer metastasizes to the brain 28 months after the initial diagnosis.
Prostate cancer metastasizes to the brain in 0.6–6% (Catane et al. 1976; Castaldo et al. 1983; Khanson and Imyanitov 2001; Tremont-Lukats et al. 2003; Hatzoglou et al. 2014). According to Catane et al. (1976), based on the analysis of diagnostic findings in a series of autopsies with MTSs to the brain (n = 1202), PrC metastases were found only in 0.8% of cases. According to Lynes et al. (1986), at autopsies of 856 cases, PrC metastases to the brain were identified in 1.3% (n = 11). When analyzing a large clinical sample (n = 6107) in patients with secondary metastatic brain lesions, Sutton et al. (1996) identified PrC metastases in 0.9%, with 0.6% of them being diagnosed in vivo. In our material, PrC metastases to the brain amounted to no more than 1% (9 patients).
Prostate cancer metastasizes to CNS via both hematogenous and mixed lymphohematogenous routes. By the lesion location in the brain structures, Kalkun (1963) noted their most frequent location in the brain substance (78%), less frequently in the dura (18%) and pia (4%) mater. According to Lynes et al. (1986), in a group of patients with prostate adenocarcinoma (n = 55), metastases were observed in the meninges in 67%, in the hemispheres in 25%, and in the cerebellum in 8%. Prostate cancer often metastasizes to the orbital bones, the greater wing of the sphenoid bone, significantly increasing the size of the orbit and causing a secondary sclerotic bone response.
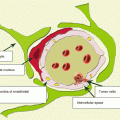
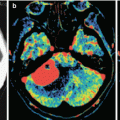
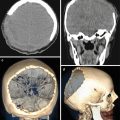
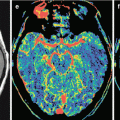
A neuroimaging picture of metastatic brain lesions in PrC does not differ from that in cerebral metastases from other organs: in most cases, a decreased density dominates on CT, a decreased MR signal is observed in native (T1-weighted) images, and an increased signal is predominant in T2-weighted images. An increase in the density and an increase in the intensity of MR signal characteristics are often associated with the presence of hemorrhages in metastatic lesions and protein-containing and calcified inclusions. Lesions often accumulate the contrast agent with a pronounced ring enhancement and an increased CT density (a high intensity of MR signal), which is explained by the presence of necrosis in the central part of the lesion and its rich vascularization on the periphery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree