Chapter 22 Prostate Cancer
Epidemiology and Risk Factors
Prostate carcinoma is the most frequently diagnosed visceral cancer and the second most common cause of cancer death among American men.1 African American and Jamaican men of African descent have the highest incidence rates in the world. Geographically, the disease is more common in North America and northwestern Europe than in Asia and South America.2 With the advent of a method to potentially detect the disease using PSA blood testing, the incidence of prostate cancer increased rapidly between 1982 and 1992 and has risen less dramatically since. Greater than 70% of cases are diagnosed in men older than age 65. Since 1995, the incidence has risen less sharply in men younger than 65 and leveled off in men older than 65 years old,2 suggesting that the impact of PSA on the detection of prostate cancer in the population has leveled off. The use of PSA for screening is currently controversial. Ethnicity can influence one’s chances of both getting and surviving the disease. In the United States, African American men have both a higher incidence of and more than twice the death rate from prostate cancer than whites. Fortunately, death rates from prostate cancer have been declining, particularly since the mid 1990s. Some of the decline may be attributed to earlier diagnosis and therapeutic intervention. Five-year survival is effectively 100% when disease is local or regional, but drops to 32% with distant metastases. For all stages, survival is 99% at 5 years, 93% at 10 years, and 79% at 15 years.2
Established risk factors include age, ethnicity, and family history. The latter is a contributor in only 5% to 10% of cases,2 and the risk increases with a greater number of first-degree relatives affected and younger age of diagnosis in those relatives. Furthermore, patients with a family history tend to be diagnosed with prostate cancer at an earlier age.3 Environmental factors, including diets high in saturated fat, may increase risk, and obesity may increase the risk of dying from the disease.4 An antioxidant, lycopene, may reduce risk. It is found in red and pink foods such as tomatoes.2
Anatomy and Histology
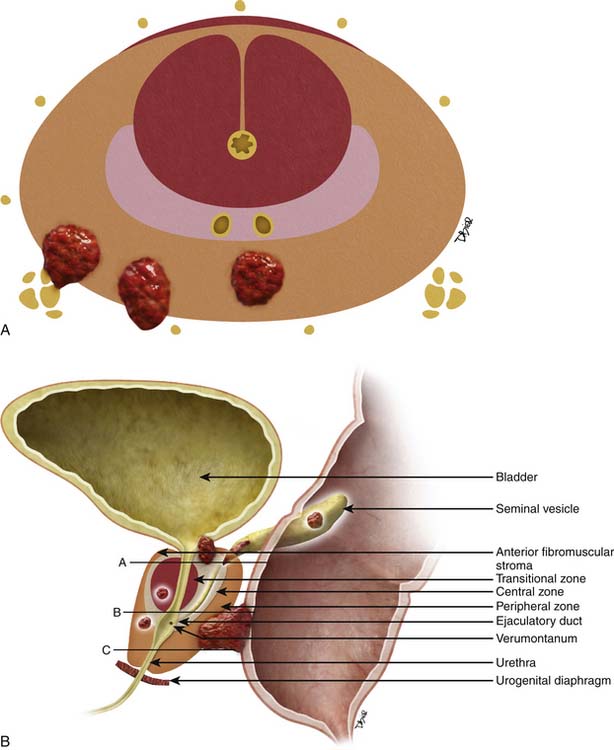
The prostate gland is bounded superiorly at its base by the urinary bladder and inferiorly by the urogenital membrane. It is encircled by the levator ani bilaterally, the symphysis pubis anteriorly, and the rectum posteriorly. In the model by McNeal and coworkers,5 the prostate gland is divided into four zones—the transition zone, the central zone, the peripheral zone, and the nonglandular anterior fibromuscular stroma (Figure 22-1)—that contain 5%, 20%, 70% to 80%, and 0% of glandular tissue and 25%, 5%, 70%, and 0% of prostate cancers, respectively. Cancers resolvable by conventional imaging are essentially within the peripheral zone. The transition zone is most susceptible to age-related benign prostatic hyperplasia (BPH). Normally, it composes only 5% to 10% of the total glandular tissue of the prostate, but in older men with BPH, it can compose the majority of prostate tissue. The transition zone cannot be separated from the central zone by imaging, and on images, the two together are often referred to as the central gland or zone. The central zone is cone shaped, with its widest portion at the base of the bladder and its apex at the verumontanum. The seminal vesicles are located posterior and superior to the prostate gland and give off the ejaculatory ducts, which traverse the central zone, providing a conduit for seminal fluid from the seminal vesicles to the urethra. The relative amount of peripheral zone to central zone increases from the base of the gland to the apex of the gland. Inferiorly, the peripheral zone comprises all of the prostatic glandular tissue at the apex, and superiorly, it invests the posterior portion of the central zone.
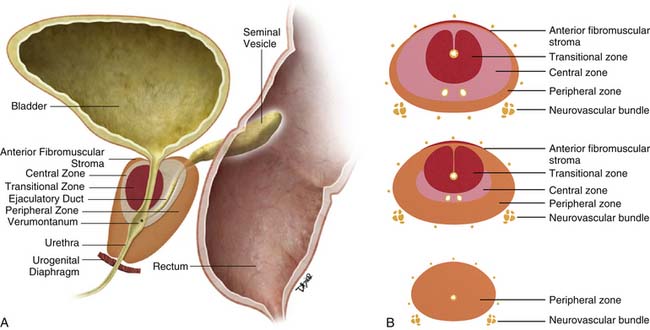
Figure 22-1 Prostate gland anatomy: A, Sagittal view. B, Axial view: top, base; middle, mid gland; bottom, apex.
The prostate gland is partially invested by a coalition of fibrous tissue, historically called the “capsule,” that is most apparent posteriorly and posterolaterally. The capsule is an important landmark for assessing extraprostatic tumor extension (EPE). The neurovascular bundles course posterolateral to the prostate capsule bilaterally at 5 and 7 o’clock (see Figure 22-1). At the apex and the base, the bundles send penetrating branches through the capsule, providing a route for EPE. The normal fat plane between the prostate gland and the neurovascular bundles seen on imaging is lost when there is neurovascular involvement by prostate carcinoma. Whereas perineural invasion by prostate cancer on biopsies does not seem to correlate with positive margins or poorer survival, it may be indicative of EPE.6,7 Identifying tumor involvement of the neurovascular bundles is important when planning for nerve-sparing prostatectomy. The neurovascular bundles run within the substance of the parietal pelvic fascia, also termed the lateral prostatic fascia. During nerve-sparing radical prostatectomy, this fascia is incised anterior to the bundles and reflected off the prostate capsule.
Histologically, 95% of prostate cancers are adenocarcinomas that develop from the acini of the prostatic ducts. Other histologic types include small cell carcinoma (the most common variant); mucinous, ductal, squamous, sarcomatoid, and transitional cell carcinomas; adenoid basal cell tumors; and malignant mesenchymal tumors.8
In the United States, histologic evaluation of prostate adenocarcinomas is performed using the Gleason grading system. Tumors are assigned a primary grade on the basis of the predominant pattern of differentiation and a secondary grade on the basis of the second most common pattern. The two numbers are added to produce a Gleason score. For example, a tumor described as “Gleason grade 3 + 4 or 4 + 3” will have a Gleason score of “7.”9 The biologic behavior of a Gleason score 4 + 3 is more aggressive than 3 + 4 regardless of the number of cores.10
Cancers with Gleason scores of 6 or lower are considered well-differentiated and are associated with a good prognosis. Those with a Gleason score of 8 to 10 have the worst prognosis and the highest risk for recurrence.11 Tumors with Gleason scores of 7 have a variable prognosis and an intermediate risk of recurrence. Serum PSA is not useful in uncommon high-grade tumors that do not produce PSA.11 Prostate cancer tends to be multifocal and the Gleason score at biopsy may differ from that obtained at surgery.12
Key Points Anatomy and Histology
• The prostate is divided into the central zone and the peripheral zone. By imaging, the peripheral zone is better evaluable for tumor.
• The base of the gland is near the bladder neck, and the apex is near the urogenital diaphragm.
• The neurovascular bundles are at 5 and 7 o’clock.
• Histology is defined by the Gleason score, which consists of two numbers added. The predominant type goes first, for example, 4 + 3 = 7 Gleason score with the predominant type being 4.
Clinical Presentation
At presentation, early prostate cancer is commonly asymptomatic. When present, local symptoms include difficulty starting or stopping urine flow; painful, weak, or interrupted urine flow; increased frequency; and hematuria. Most of these symptoms may be due to coexistent BPH. A large prostate cancer or a smaller prostate cancer in the transition zone affecting the urethra may cause similar symptoms. The rapid acuity of presentation usually prompts the consideration for cancer; in comparison, the onset is insidious and chronic with BPH. Back, pelvis, or thigh pain may indicate distant metastatic disease. Unfortunately, the symptoms are not specific to prostate cancer and can be mimicked by a variety of benign conditions.2 Prostate cancer may be suspected by elevated PSA, digital rectal examination (DRE), or imaging.
Prostate carcinoma is often suspected when an abnormal DRE is noted by physical examination or the serum PSA is elevated. Interobserver agreement among urologists is only fair for detecting malignancy by DRE and DRE is not accurate for staging.13,14 For serum PSA, no specific cutoff point simultaneously provides optimal sensitivity and specificity; instead, it provides a spectrum of risk at all ranges.15 Although a serum PSA level greater than 4 ng/mL has traditionally been considered abnormal, almost 27% of biopsy-proven prostate cancers present with “normal” PSA, and 70% to 80% of patients with “elevated” PSA (>4 ng/mL) do not have prostate carcinoma.16,17 The chance of prostate carcinoma increases with increasing PSA.18
There is controversy regarding using PSA to screen for prostate cancer. If performed after a discussion between the patient and the physician of the risks and benefits of screening, the American Cancer Society (ACS) guidelines for early detection of prostate cancer include annual screening by DRE and serum PSA of men age 50 or older with a 10-year life expectancy. Evaluation may begin at age 40 to 45 for high-risk individuals, such as African Americans and/or patients with a first-degree relative diagnosed with the disease at a young age.2 Although the role of early detection with PSA has been questioned, recent findings suggest that early detection and active treatment were important in the recent lowering of death rates, with the greatest survival impact on younger (age < 65 yr) men.1,19 However, PSA screening has also been cited as a cause of overdiagnosis.20,21 To improve upon traditional serum PSA, derivative tests based on PSA have been and are being developed, for example, PSA density, PSA velocity, age-specific reference range, PSA isoforms, and percent-free PSA.22 Non–PSA-based tests may also improve the diagnostic accuracy of markers, for example, urinary PCA3,23 a noncoding mRNA rarely expressed in benign prostate tissue but overexpressed in the majority of prostate cancer. Incidental findings (e.g., an enlarged prostate, abnormal T2 signal on MRI, or gross features of local invasion, pelvic lymphadenopathy, or sclerotic bone metastasis) on imaging obtained for another reason may initiate workup for prostate cancer.
Key Points Clinical Presentation
• The patient is often asymptomatic or may have nonspecific symptoms such as related to voiding for local disease or bone pain for metastatic disease.
• Controversy exists regarding PSA for routine screening. Derivative and new tests are being developed.
• PSA is useful for monitoring patients diagnosed with prostate cancer.
Patterns of Tumor Spread
Prostate cancer tends to spread by local extension (Figure 22-2). EPE tends to be seen at 5 and 7 o’clock, where the neurovascular bundles send penetrating branches through the capsule. This also provides a route for neurovascular bundle involvement. Further lateral growth may involve the levator ani and then the pelvic sidewalls. Tumor may also spread superiorly to involve the seminal vesicles—either by direct extension or via the ejaculatory ducts—or the bladder, by direct extension or via the urethra. Superior and inferior growth may also involve the two sphincters for micturation, superiorly at the base of the bladder and inferiorly at the urogenital diaphragm. Rarely, the tumor may grow inferiorly along the urethra beyond the membranous urethra. Posteriorly and uncommonly, the tumor may involve the rectum.
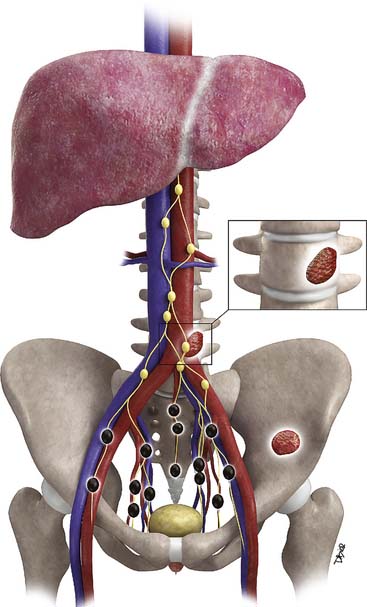
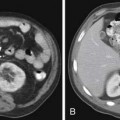
Metastases to regional lymph nodes (Figure 22-3) occurs most commonly along the obturator chain (medial to external iliac area) but also occurs along other regional nodes such as along the internal iliac, external iliac, and presacral chains.24 Lymph node metastases may also occur along the common iliac and retroperitoneum chains. Skip metastases to the retroperitoneum may occur in the absence of pelvic lymphatic metastasis. After lymph node resection or radiation therapy to the pelvis, recurrence may occur particularly in retroperitoneal nodes outside the treated field. Hematogenous metastases (see Figure 22-3) tend to occur primarily to the skeleton and are usually blastic. Lytic lesions may be seen with advanced disease. Occasionally, spinal metastases may cause cord compression. Other visceral metastases are associated with advanced disease and are rare; if found, such metastases may suggest less differentiated forms of prostate cancer or another primary cancer.
Key Points Tumor Spread
• EPE most commonly occurs at 5 and 7 o’clock; therefore, it can involve the neurovascular bundles.
• Seminal vesicles or other adjacent organs such as bladder and rectum are less commonly involved.
• Lymphatic metastases are usually to intrapelvic lymph nodes, but skip metastases to retroperitoneal lymph nodes may occur.
• Visceral metastases are most commonly to bone and are most commonly sclerotic.
Staging
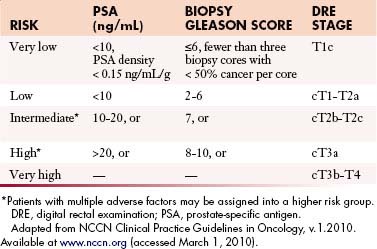
The primary goals of staging (Table 22-1) are, in general, to allow for risk stratification and allocation to reasonable treatment strategies. Specifically, this involves distinguishing patients with organ-confined, locally invasive, or metastatic disease and assessing risk of treatment failure. Risk-stratification guides therapy and integrates PSA; tumor-node-metastasis (TNM) clinical staging; and biopsy data including Gleason score, number of cores positive, and amount of cancer in each core (Table 22-2).
Table 22-1 Tumor-Node-Metastasis Staging System for Prostate Cancer
| Primary Tumor (T) | |
| Clinical | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Clinically inapparent tumor neither palpable nor visible by imaging |
| T1a | Tumor incidental histologic finding in ≤ 5% of tissue resected |
| T1b | Tumor incidental histologic finding in > 5% of tissue resected |
| T1c | Tumor identified by needle biopsy (e.g., because of elevated PSA)* |
| ——— For evaluating intraprostatic disease, imaging becomes more useful ——— | |
| (Primarily MRI) | |
| T2 | Tumor confined within the prostate |
| T2a | Tumor involves one half of one lobe or less |
| T2b | Tumor involves more than one half of one lobe, but not both lobes |
| T2c | Tumor invades both lobes |
| ——— For evaluating local extension, imaging becomes more useful ——— | |
| (Primarily MRI and sometimes CT) | |
| T3 | Tumor extends through the prostate capsule† |
| T3a | Extracapsular extension (unilateral or bilateral) |
| T3b | Tumor invades seminal vesicle(s) |
| T4 | Tumor is fixed or invades adjacent structures other than seminal vesicles: bladder neck, external sphincter, rectum, levator muscles, or pelvic wall |
| Pathologic (pT) | |
| pT2 | Organ confined‡ |
| pT2a | Unilateral, involving one half of one lobe or less |
| pT2b | Unilateral, involving more than one half of one lobe, but not both lobes |
| pT2c | Bilateral disease |
| pT3 | Extraprostatic extension |
| pT3a | Extraprostatic extension or microscopic invasion into the bladder neck§ |
| pT3b | Seminal vesicle invasion |
| pT4 | Invasion of bladder (gross), rectum, levator muscles, and/or pelvic sidewall |
| Regional Lymph Nodes (N) | |
| Clinical | |
| Nx | Regional lymph nodes were not assessed |
| N0 | No regional lymph node metastasis |
| ——– For staging and guiding lymph node biopsies, imaging becomes more useful ——– | |
| (Primarily CT and MRI) | |
| N1 | Metastasis in regional node(s) |
| Pathologic | |
| PNx | Regional lymph nodes not sampled |
| pN0 | No positive regional nodes |
| pN1 | Metastases in regional node(s) |
Distant Metastasis (M) | |
| Mx | Distant metastasis cannot be assessed (not evaluated by any modality) |
| M0 | No distant metastasis |
| ——— For staging metastatic disease, imaging becomes more useful ——— | |
| (Multiple modalities, radiographs, CT, bone scan, MRI) | |
| M1 | |
| M1a | Nonregional lymph node(s) |
| M1b | Bone(s) |
| M1c | Other site(s) with or without bone disease |
CT, computed tomography; MRI, magnetic resonance imaging; PSA, prostate-specific antigen.
* Tumor found in one or both lobes by needle biopsy, but not palpable or reliably visible by imaging, is classified as T1c.
† Invasion into the prostatic apex or into (but not beyond) the prostate capsule is classified not as T3 but as T2.
‡ There is no pathologic T1 classification.
§ Positive surgical margins should be indicated by an R1 descriptor (residual microscopic disease).
 When more than one site of metastasis is present, the most advanced category is used. pM1c is the most advanced.
When more than one site of metastasis is present, the most advanced category is used. pM1c is the most advanced.
Adapted from Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010:525.
Local Staging (T)
Although local tumor staging (see Figure 22-2) is based upon findings from DRE, imaging can be beneficial. Stage T1 describes incidental detection of cancer. For example, stages T1a and T1b may be noted in specimens from transurethral resection of the prostate (TURP) performed for BPH. Stage T1c describes prostate cancer detected by elevated PSA, but not palpable by DRE. Cancer palpable by DRE or imaged on transrectal ultrasonography (TRUS) but confined within the prostatic capsule is considered T2. Cancer extending beyond the capsule is considered T3. This latter group includes EPE, periprostatic neurovascular involvement, and/or seminal vesicle involvement. Stage T4 describes tumor extending to other adjacent structures.25
Metastatic Disease (N and M Staging)
Prostate cancer may metastasize by lymphatic or hematogenous routes. The incidence of metastatic disease is extremely low in patients with stage T1-2 disease, serum PSA less than 20 ng/mL, and Gleason score less than 8.26 Thus, metastatic workup is usually reserved for patients with a Gleason score greater than 7, serum PSA greater than 20 ng/mL, stage greater than T2, or symptoms suggestive of metastasis.26
Metastasis (see Figure 22-3) to regional lymph nodes occurs primarily along the obturator, internal iliac, common iliac, and presacral chains. Metastasis to inguinal lymph nodes is exceedingly rare. It has been suggested that lymph node metastasis occurs stepwise from the pelvis to the retroperitoneum27; however, there are reports that up to 50% of nodal metastases can be para-aortic without a concurrent pelvic nodal metastasis, suggesting hematogenous rather than lymphatic spread.28 Such hematogenous spread potentially occurs via Bateson’s plexus. Importantly, at staging, nodal disease within the pelvis is considered regional nodal metastasis (N1); whereas nodal disease in the common iliac chains and retroperitoneum is considered distant metastasis (M1a). This, as well as the propensity of prostate cancer to metastasize to the lumbar spine, suggests that including the abdomen may be helpful when performing prostate MRI or this area may be evaluated by CT if abdominal CT is already to be performed in the workup of the patient.29
Hematogenous metastasis to bone occurs most frequently to the lumbar spine, pelvis, ribs, and femoral heads (M1b). In advanced disease (M1c), viscera more commonly involved include the liver, adrenal glands, and lungs.25,26
Key Points Staging
• T2 lesions may be visualized by imaging, most commonly by MRI.
• T3 and T4 disease may be visualized by CT and MRI.
• T3 includes EPE and microscopic invasion of the bladder neck and seminal vesicles.
• Regional lymph nodes encompass N1.
• M1a includes nonregional lymph nodes including along the common iliac chain and retroperitoneum.
• Metastases commonly occur to bone whereas visceral organ metastases are rare.
Imaging
Imaging in Local Staging
Transrectal Ultrasound
In patients suspected to have prostate carcinoma, diagnosis is generally made by TRUS-guided biopsy. Because ultrasound has limited sensitivity to distinguish prostate tumors, systematic biopsy is used. Traditionally, it employed a sextant (six site) approach in which a parasagittal plane guided three cores of each lobe (base, mid gland, and apex), yielding an approximately 25% cancer detection rate when serum PSA was between 4 and 20 ng/mL.30 Repeat biopsy demonstrated cancer in approximately 20% of men with persistently elevated serum PSA and negative initial biopsy.31,32 Thus, more cores were tried. Presti and colleagues33 achieved approximately 40% yield using a 10-core approach; even more cores have been advocated34 to minimize sampling errors.
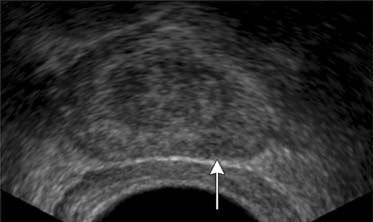
TRUS utilizes a high-frequency transducer (7-10 MHz) to delineate zonal anatomy, but it cannot reliably differentiate between benign and malignant prostate tissue. Tumors have varying appearances on TRUS: approximately 40% to 50% are hypoechoic (Figure 22-4), 40% are isoechoic, and others are hyperechoic.35 Confounding lesions can also present as hypoechoic lesions, for example, prostatitis, atrophy, prostatic epithelial neoplasia, and ductal ectasia.36 Lee and associates35 noted that the positive predictive value for cancer of a hypoechoic area in the peripheral zone alone was 41%, 52% if PSA was greater than 4 ng/mL, and greater than 71% if there was also an abnormal DRE.
Vascular imaging using color or power Doppler ultrasound may demonstrate lesions not visualized by conventional gray-scale ultrasound; however, such lesions are not specific and the new techniques do not perform as well as systematic biopsy in diagnosing prostate cancer.37 Although new intravenous sonographic contrast agents can improve sensitivity for prostate cancer compared with gray-scale imaging from 38% to 69%, the role of contrast-enhanced ultrasound has yet to be determined. It has been reported that TRUS can detect EPE, but accuracy remains suboptimal.38,39
Magnetic Resonance Imaging
EPE is more accurately diagnosed by TRUS or endorectal MRI than by DRE.39 Most studies have shown that high-resolution endorectal MRI provides higher accuracy in staging local disease than does TRUS.40–42 Moreover, adding data from endorectal MRI to Partin nomograms improves prediction of organ-confined prostate cancer versus extraprostatic disease at radical prostatectomy (area under receiver operating curve [ROC] curve 0.81 vs. 0.90, nomogram vs. nomogram plus MRI) in all risk groups, with greatest impact on intermediate- and high-risk groups.43 In the study by Wang and coworkers,43 extraprostatic disease was defined on MRI by extracapsular extension, seminal vesicle invasion, or lymph node metastasis; no statistically significant improvement was noted by the addition of spectroscopy. MRI also allows simultaneous screening of the regional lymph nodes and pelvic bones.
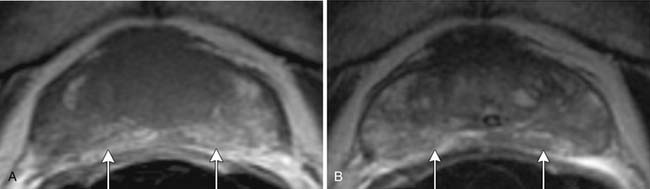
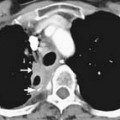
T1-weighted axial images are employed to screen the pelvic nodes and bones for metastasis and to identify hemorrhage in the prostate gland. Zonal anatomy is best seen on T2-weighted images (T2WI) (Figure 22-5), but cannot be distinguished on T1-weighted imaging (T1WI). Typically, the central gland is heterogeneous on T2WI owing to BPH and is isointense on T1WI. In the central zone, it is difficult to separate cancer from the BPH that invariably occurs in this age group. In comparison, the peripheral zone is relatively homogeneous and hyperintense on T2WI and isointense on T1WI. Located at 5 and 7 o’clock, the neurovascular bundles appear as oval in axial and linear in long axis, hypointense structures surrounded by hyperintense fat on T1WI. Periprostatic vessels appear as serpiginous or honeycomb iso- to hyperintense structures on T2WI (Figure 22-6) and should be identified owing to the possibility of hemorrhage at the time of surgery.
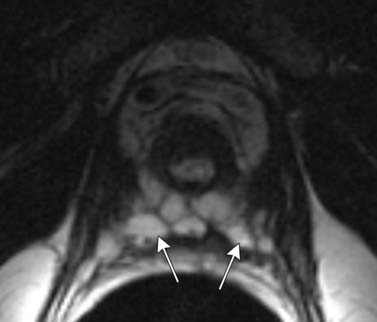
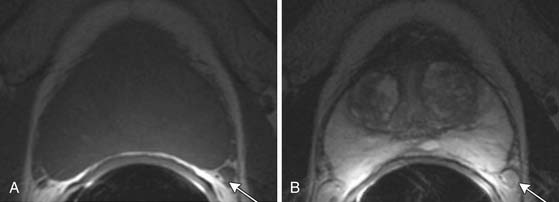
Figure 22-6 Axial MRI demonstrates periprostatic blood vessels (arrows) adjacent to the prostate apex.
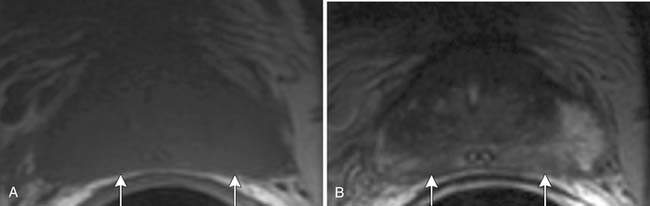
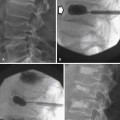
In the peripheral zone, prostate tumors appear hypointense on T2WI and isointense on T1WI (Figure 22-7). However, low T2-weighted signal is not specific for cancer. Underlying hemorrhage secondary to biopsy (Figure 22-8), prostatitis, atrophy, or posttreatment change can present with low signal intensity. MRI is generally performed at least 6 weeks after prostate biopsy to allow resolution of hemorrhage, which has become more problematic with extended numbers of core biopsies. Fortunately, hemorrhage can be identified as high signal on T1WI. Unfortunately, areas of high T1 signal compromise detection of tumor in the prostate on T2WIs. Performing axial T1WI and T2WI with the same slice thickness and field of view allows alignment of the two sequences that can aide distinguishing tumor separate from hemorrhage. Using MRI can reduce the number of false-negative biopsies in patients with a previous negative biopsy (negative predictive value of 84-91% and accuracy of 77-78%).44
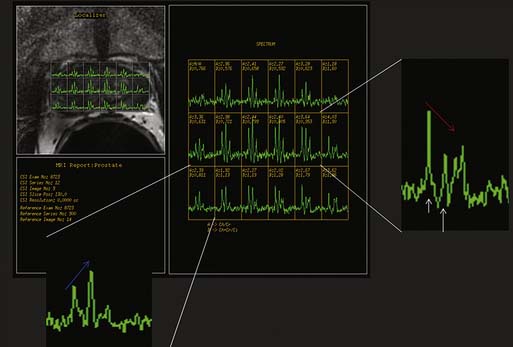
Magnetic resonance spectroscopic imaging (MRSI) with an endorectal coil has been used to improve tumor detection. MRSI is a metabolic imaging technique, and for prostate cancer, the most commonly studied markers include choline, creatine, and citrate; others include polyamines, lipids, and lysine.45 Most commonly, prostate cancer is identified by increased choline (a byproduct of increased cell membrane metabolism) and decreased citrate, a normal product of prostate metabolism (Figure 22-9). In conjunction with endorectal MRI, MRSI has been reported to provide sensitivity and specificity for prostate cancer as high as 95% and 91%, respectively, and to provide similar accuracy to sextant biopsy for intraprostatic tumor localization, except at the apex where MRI with MRSI was more accurate than biopsy.46,47 MRSI may also predict tumor aggressiveness.48 However, obtaining optimal spectral resolution and interpreting MRSI remains challenging, and efficacy compared with T2WI or other functional techniques is unclear. Drawbacks of MRSI include relatively poor spatial resolution (~4 mm), technical demand, artifacts, inability to directly predict the periprostatic area, and requirement of specialized software and expertise in acquiring and interpreting the data.
The main roles of MRI in prostate cancer currently are local staging of tumors in patients with an intermediate risk for treatment failure11 and assessing prostate size. The formula for a prolate ellipse is commonly used (anteroposterior × transverse × cephalocaudal × 0.52) to calculate prostate volume. Jager and colleagues49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree