Chapter 33 Bone Metastases
Introduction
Bone metastases are common in patients with advanced malignancies. Autopsy series have shown an incidence of approximately 70% in breast and prostate cancer and 35% in lung cancer.1,2 Osseous metastases can profoundly influence quality of life and prognosis. Early and accurate detection is important for therapeutic planning, and many imaging modalities can be used for this purpose. X-ray–based technologies such as radiography and computed tomography (CT) image bone calcium, skeletal scintigraphy (SS; bone scan) detects bone formation, magnetic resonance imaging (MRI) images the soft tissue of the marrow cavity, and positron-emission tomography (PET) reflects the glucose metabolism of the lesions. The therapeutic response of bony metastases can be assessed through means of response criteria that are also used to determine the clinical efficacy of cancer therapy. Response criteria developed at the M. D. Anderson Cancer Center (MDA criteria) are useful for interpreting the behavior of bone metastases.3,4 Successful new therapies are prolonging the lives of cancer patients with a concomitant increase in skeletal metastasis–related complications such as pain, pathologic fractures, and spinal cord compression.2,5 Patients with multiple painful bone metastases can be treated with systemic therapy such as bisphosphonates or radiopharmaceuticals. When symptomatic lesions are limited in number, they can be treated with radiation therapy, surgery, or percutaneous techniques such as vertebroplasty or radiofrequency ablation (RFA). This chapter discusses the role of imaging in the detection and therapeutic response of bony metastases with a discussion of treatment including topics such as medical therapy, radiation, surgery, and percutaneous procedures.
Distribution and Pathophysiology
Cortical bone is composed of compact, lamellar bone that provides structural support and forms a heavily calcified, well-defined boundary for the marrow cavity. The marrow contains delicate trabecular bone and either red, hematopoietic or yellow, fatty marrow. The majority of bone metastases are hematogenous and may spread by arterial or venous routes. Venous backflow to the spine and pelvis can occur through a relatively valveless network of blood vessels called Batson’s vertebral plexus following Valsalva episodes of increased abdominopelvic or intrathoracic pressure.6,7 Skip metastases are uncommon, occurring most frequently in osteosarcoma and rarely in patients with Ewing’s sarcoma.8,9 They are defined as lesions that are anatomically separate from a primary bone malignancy but arise either in the same bone or directly across an adjacent joint in the absence of other bony metastases. Enneking and Kagan10 proposed that skip metastases could arise in the same bone through invasion of the venous sinusoids of the marrow cavity and that transarticular spread could occur through venous backflow, similar to the mechanism involving Batson’s plexus.
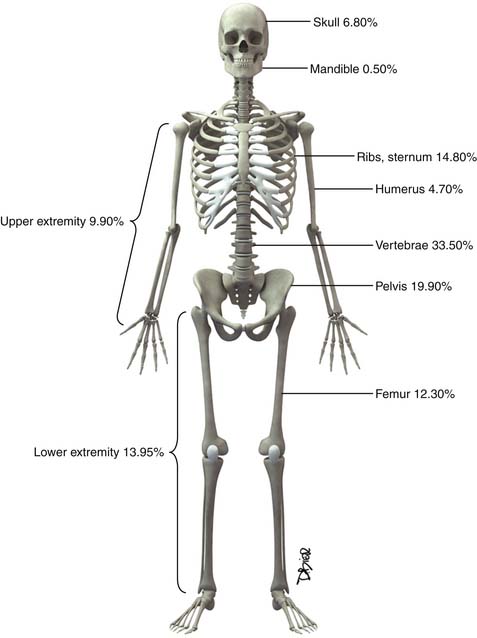
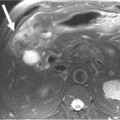
Following the distribution of highly vascular red marrow, osseous metastases occur most commonly in the medullary cavity of the bones of the axial skeleton. In a study of 4105 bone metastases in 2001 patients, Clain11 reported the highest incidence of lesions in the vertebrae (33.5%), pelvis (19.9%), ribs (12.2%), femora (12.3%), skull (6.8%), and humeri (4.7%) (Figure 33-1).
Bone metastases can be classified as lytic, blastic, or mixed depending on the activity of tumor-stimulated host osteoclasts and osteoblasts.12 Osteoclasts are large, multinucleated cells with a specialized cell membrane (the “ruffled border”) that resorb bone, and osteoblasts are smaller, mononucleated cells that form new bone.13 These cells produce lytic or blastic lesions, respectively, and a combination of the two processes results in mixed lesions. Microscopically, the tumor cells responsible for their activation do not need to be in the immediate vicinity of the osteoclasts and osteoblasts. The complex biochemical processes involved in the formation of bone metastases have been well reviewed in other sources14 and constitute an area of rapidly evolving cancer research that is beyond the scope of this chapter.
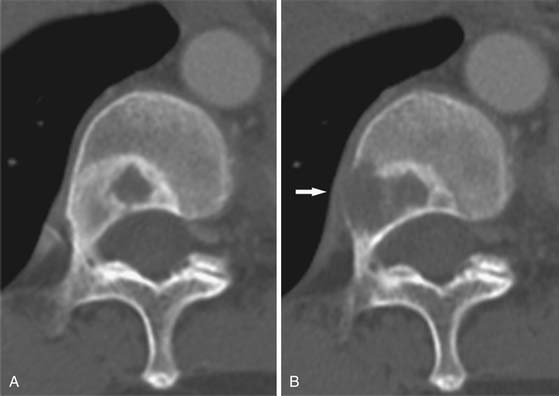
The appearance of untreated bony metastases can be predicted by the histology of the primary tumor. Of the most common primaries to metastasize to bone,2 prostate carcinoma most frequently produces blastic metastases; breast and lung cancer are predominantly lytic but can be mixed or blastic; and tumors such as renal cell carcinoma and thyroid carcinoma produce lytic metastases. Lesion margins can be well-defined, ill-defined, or expansile (Figure 33-2). Unlike primary bone tumors that typically demonstrate nonaggressive margins when benign and aggressive margins when malignant,15 virtually all bone metastases are malignant regardless of the appearance of the margins. Metastases from solid malignancies and myeloma are the most common bone tumors to arise in patients older than 40 years.
Detection
Conventional Radiography
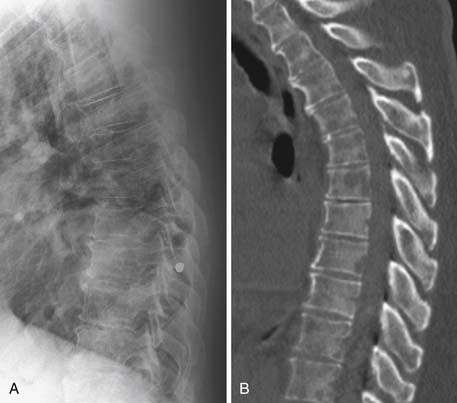
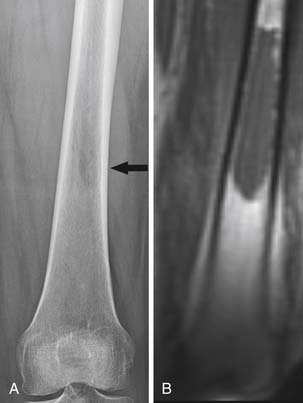
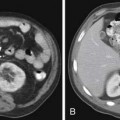
Radiography, CT, bone scan, single-photon emission tomography with integrated CT (SPECT/CT), MRI, and PET with integrated CT (PET/CT) are methods used to detect bone metastases and evaluate their response to treatment. Radiography, like all x-ray–based technologies, primarily images bone calcium and the majority of the calcium is found in the cortex. Radiography is specific but not sensitive for the detection of bony metastases.16 A minimum of 30% to 50% bone loss must occur for most lytic metastases to be detectable on radiographs (XRs),17,18 and metastases in the axial skeleton are often obscured by overlapping anatomic structures, such as bowel content or other bones (Figure 33-3). Nevertheless, the high specificity and low cost of radiography make it an ideal initial imaging modality for the detection of bony metastases in areas of focal bone pain.3 Radiography is also the optimal imaging modality to assess for pathologic fracture in the appendicular skeleton where no overlapping structures obscure the lesions.19
Computed Tomography
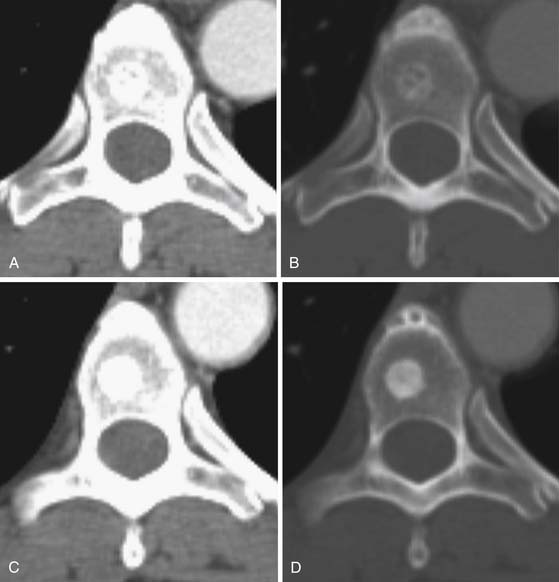
CT is an excellent modality for detailed imaging of mineralized structures such as bone or calcifications.20,21 CT is useful for determining the extent of cortical bone destruction in irregular bones such as the vertebrae, and the cross-sectional nature of CT eliminates the effect of overlapping structures when assessing the bony detail of the axial skeleton (see Figure 33-3). Owing to limited soft tissue contrast resolution, this modality is not the best choice for imaging the marrow cavity. Whereas radiography is the optimal method for evaluating the structural integrity of the long bones of the appendicular skeleton, CT is often the best choice for making the same determinations in the axial skeleton. For example, the cortical detail seen on CT of the spine (with sagittal and coronal re-formations) can provide an estimate of spinal stability (Figure 33-4). Bone windowing displays a greater degree of bony detail than soft tissue windowing and is recommended for the detection and follow-up of bone lesions (Figure 33-5).
Nuclear Medicine
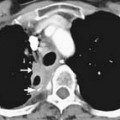
Technetium-99m methylene diphosphonate (MDP) is one of the most commonly used tracers for SS and its mechanism of action involves a complex interaction of bone repair and blood flow. SS targets the bony cortex, binding to the hydroxyapetite produced when the bone attempts to repair damage caused by metastases.22 As a whole body imaging modality, bone scan is currently the method of choice for detecting asymptomatic osseous metastases during initial staging or restaging of patients with malignancies that produce blastic or mixed bone metastases, such as prostate, breast, and lung cancer.19 Lytic metastases may not allow sufficient reparative bone formation for detection on SS (see Figure 33-4). Therefore, the radiographic skeletal survey can be used in place of or in addition to bone scan for staging malignancies that produce purely lytic metastases such as renal cell and thyroid carcinoma. Myeloma lesions suppress osteoblast activity, producing false-negative results to the extent that bone scan is not recommended for staging these patients.23–25
Bone scans are more sensitive than specific for the detection of osseous metastases.16 A wide range of benign findings (e.g., arthritis, healing fractures, benign bone tumors such as enchondromas and fibrous dysplasia) can induce tracer uptake and mimic metastatic disease.26 Alternatively, metastases that heal with sclerosis can subsequently accumulate more radionuclide than was present during disease progression, resulting in a deceptive “flare” phenomenon.27,28 Because bone scans provide limited anatomic detail, these pitfalls cannot be resolved by SS alone. Therefore, the sensitivity of bone scan is often combined with the specificity of radiography to provide a powerful and relatively inexpensive means of diagnosing indeterminate findings on bone scan.3,19,26
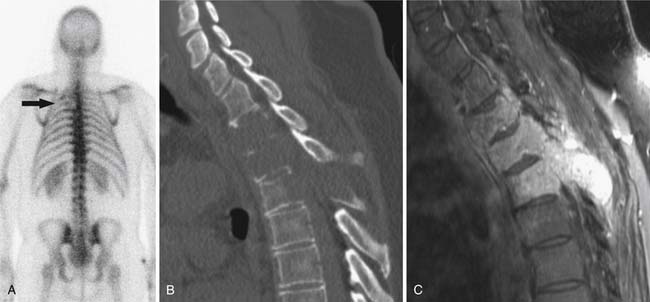
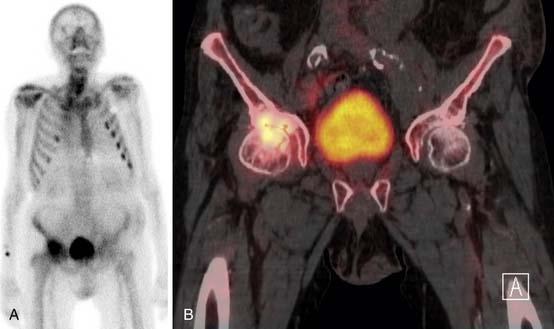
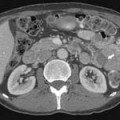
SPECT/CT is a dual-modality imaging technique that acquires scintigraphic and CT data sets on a single, hybrid scanner. Traditional bone scanning is a planar modality that produces single anterior and posterior whole body images with optional oblique or spot views. SPECT is used to perform bone scans in a cross-sectional manner. The axial images increase contrast resolution, resulting in greater sensitivity29 and specificity30 for detection of bony metastases than those achieved with planar scans. Nevertheless, anatomic detail remains limited and specificity of SPECT is further increased when fused with CT.31 Modern SPECT/CT scanners are multifunctional machines that can perform planar bone scan, SPECT, and CT on the same hybrid scanner. SPECT/CT is most commonly used to determine the etiology of indeterminate uptake on planar bone scan (Figure 33-6), which can be accomplished in the same imaging session and with the same dose of tracer on the hybrid scanner.
[18F]-fluoro-2-deoxy-D-glucose (FDG) PET is a functional imaging modality that reflects cellular glucose metabolism. FDG, the most commonly used PET tracer for oncologic indications, is a radiolabeled glucose molecule. Numerous malignancies have high glucose metabolism. Two important factors regarding the uptake and intracellular accumulation of FDG involve cell membrane glucose transport proteins (GLUTs) and the intracellular enzyme hexokinase. Of the GLUT family of transport proteins, GLUT 1 is both ubiquitous and up-regulated in many neoplasms. Once FDG enters the cell, it is phosphorylated by hexokinase into FDG-6-phosphate.32 This molecule is not suitable for further metabolism; the negative charge imputed by the phosphate moiety makes it impermeable to the cell membrane and dephosphorylation is slow. Therefore, FDG accumulates in the cells. The tracer emits positrons that, following an interaction with an electron, results in the near-simultaneous emission of a pair of 511-keV photons (nearly 180 degrees apart) that are detected by scintillation crystals embedded in the PET scanners, thereby localizing the decay event within the body.33
FDG uptake is not limited to tumor cells; other conditions result in FDG accumulation such as inflammatory (e.g., arthritis, infection) or physiologic (e.g., brain and liver function) processes. Despite these limitations, PET alone (no CT) was found to be more specific for detection of bony metastases than planar SS34–36 owing to the higher spatial resolution of PET.34,37,38 Evaluation of the morphology of the bone metastases has shown that PET reveals more lytic than blastic lesions.39,40 Lytic bone metastases typically heal with sclerosis41 and prior treatment likely influenced this conclusion in some studies. Otherwise, it has been suggested that blastic metastases may not be as easily detected by PET owing to relatively lower cellularity42 in comparison with more highly cellular lytic lesions. Current technology employs fused PET/CT data sets obtained on hybrid PET/CT scanners. A review that compared the reported sensitivity and specificity data of 10 studies that used PET alone with 2 studies that used PET/CT to detect bony metastases found the median sensitivity of PET/CT to be higher than PET (95.2%, and 83.9%, respectively) whereas the specificity was similar (94.6% and 95.8%, respectively).43 More research is needed to evaluate the influence of fused CT on the sensitivity and specificity of PET for the detection of bone metastases in general and blastic lesions in particular. Whereas PET scans are expensive and accessibility can be limited, the combination of functional and anatomic imaging makes FDG-PET/CT a powerful modality for whole body tumor assessment.
Magnetic Resonance Imaging
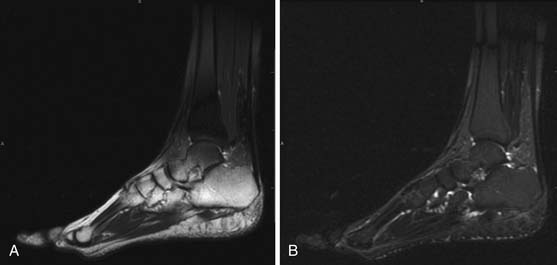
MRI is capable of providing the greatest soft tissue resolution of all imaging modalities. The optimal MRI pulse sequences for imaging the musculoskeletal system may differ from those used in body imaging. Bone movement occurs through the conscious contraction of muscles rather than through the autonomic nervous system. The majority of patients are able to remain still throughout a typical examination (~45 min), allowing the routine use of several relatively lengthy pulse sequences that permit exquisite soft tissue contrast resolution. The following pulse sequences are recommended for conventional MRI of musculoskeletal oncology: fast spin echo (FSE) T1-weighted, FSE fat-saturated (FS) T2-weighted, and FSE FS T1-weighted following intravenous gadolinium contrast. Short tau inversion recovery (STIR) can be substituted for FSE FS T2 in situations that predispose to uneven fat saturation such as when scanning irregular anatomy (foot, ankle [Figure 33-7]) or structures that are offset from the isocenter of the magnet (humeri). STIR is not used routinely owing to comparatively lower signal-to-noise ratio and resultant suboptimal image quality compared with FS T2. The metal from bony reconstructions can produce significant artifacts that can be minimized by excluding fat saturation from the contrasted sequences and replacing FS T2 with STIR.44
Most lytic and mixed bony metastases demonstrate low or intermediate T1 signal and high T2 signal and enhance. Sclerotic metastases may have a similar appearance or demonstrate low signal on all pulse sequences. Because the vast majority of skeletal metastases arise in the soft tissue of the marrow cavity, MRI allows early lesion detection by imaging the tumor while confined to the marrow.45 Conversely, radiography and CT depend primarily on the interaction of the tumor with cortex, and metastases are often visible only after the tumor has grown large enough to extend from the marrow into the cortex. MRI is also the optimal imaging modality for determining the extent of tumor in the medullary cavity21,46 and can be crucial for planning surgery or radiation therapy (Figure 33-8). Another advantage of MRI is that excellent soft tissue resolution allows detection of impending emergencies involving adjacent structures, such as major nerves, blood vessels, or the spinal cord.21,46–48 However, a disadvantage of MRI is that it cannot accurately depict cortical bone structure owing to a paucity of free protons, resulting in the black signal void typical of normal cortex. Therefore, radiography and CT are preferred for evaluating fine cortical detail. Whereas MRI has traditionally been used for limited anatomic coverage, whole body MRI is becoming increasingly feasible in the typical 1-hour patient scheduling allotment49 and may supplant bone scan and skeletal surveys as the preferred whole body screening modality for bone metastases in the future.
Future Imaging
Whole body MRI is an emerging technology that allows MRI of the entire body to be performed in a single imaging session. Conventional MRI is limited in anatomic coverage owing to a combination of the long acquisition times of traditional pulse sequences and field inhomogeneity artifacts that increase with large coverage areas. Whole body MRI has been repeatedly shown to detect more bony metastases than skeletal scintigraphy.50–54b
Considering the challenges of time limitation and artifact suppression, investigators of whole body MRI have used a variety of rapidly acquired or robust pulse sequences. Whereas some investigators have utilized T2-weighted imaging exclusively,50,55 others have sought to increase diagnostic accuracy by performing at least two complementary sequences on each patient. T2-weighted and STIR images have high sensitivity but lower specificity for the detection of osseous metastases. In contrast, T1-weighted sequences have high specificity and lower sensitivity. Lauenstein and coworkers56 found that the sensitivity and specificity of T2-weighted STIR sequences for the detection of osseous metastases to be 100% and 80%, respectively, and that the corresponding values for T1-weighted gradient recalled echo (GRE) sequences were 81% and 90%, respectively. Several investigators have taken advantage of the complementary strengths of T1- and T2-weighted MRI by performing both techniques in the same examination.52,53,57 Others have employed the same general concept by complementing T2-weighted images with an FS T1-weighted sequence obtained after administration of intravenous gadolinium contrast54 or have employed all three (T1 precontrast, T1 postcontrast, and T2-weighted sequences).58,59
In many studies, completion of multiple pulse sequences of the entire body in a reasonable time frame is facilitated by use of specialized hardware, such as a rolling table platform that utilizes a combination of stationary coils embedded in the table and a fixed anterior surface coil under which the patient moves for each anatomic station.54,56,58 In a study utilizing a rolling table platform, three sequences (T2 FSE and T1, three-dimensional GRE sequences before and after the administration of intravenous contrast) were acquired in 14.5 minutes.54 Parallel imaging techniques, based on phased-array coil technology,60 can also be used to speed image acquisition.57,61
The current trend in whole body MRI is to perform a large number of complementary pulse sequences in numerous imaging planes in approximately 1 hour or less. Dixon imaging can accomplish this objective without specialized hardware. Dixon imaging can be used to simultaneously generate three different kinds of images including T2 (no fat saturation), water-only (an FS T2 sequence), and fat-only (novel sequence in which only fat is bright) images. This has been combined with a fast T1-weighted Dixon (which again produces at least three different kinds of images in a single acquisition) and diffusion-weighted sequences to produce 56 series of images covering head, chest, abdomen/pelvis, thighs, calves, sagittal spine, and dynamic liver sequences in approximately 1 hour.49 In addition to high sensitivity for the detection of bone metastases, MRI can image soft tissues (including organs such as liver and brain), making whole body MRI a versatile imaging modality for total body imaging.54a
Whole body MRI and FDG-PET/CT are two of the newest and most comprehensive whole body imaging techniques, and research has been performed comparing their relative strengths and weaknesses for the detection of metastases. Whole body MRI has been shown to have a higher sensitivity than FDG-PET/CT for the detection of osseous metastases.43,57,58 A prospective study comparing whole body MRI and FDG-PET/CT in 98 patients found that the sensitivity of whole body MRI was greater than FDG-PET/CT for the detection of osseous and hepatic metastases, whereas whole body MRI had lower sensitivity than FDG-PET/CT for the detection of pulmonary metastases.58 In addition, FDG-PET/CT was found to be more accurate than whole body MRI for primary tumor and nodal staging. These findings suggest that the two modalities are complementary and that one of the major strengths of whole body MRI is in the evaluation of bony metastases. Future research may show that a combination of both examinations in the setting of FDG-avid tumors provides comprehensive total body imaging to the extent that few additional imaging studies are needed.
Key Points Detection
• Radiography has high specificity but low sensitivity.
• Combining radiography with the high sensitivity of bone scan, the two modalities are effective for detecting bone metastases.
• MRI effectively detects early metastases confined to the marrow, extent of disease in marrow, soft tissue extension from bone, and epidural extension.
• CT combined with SPECT and PET imaging improves lesion detection when compared with SPECT or PET alone.
• PET detects more lytic than blastic metastases, the opposite of bone scan.
Response to Therapy
Cancer response criteria are used to determine the efficacy of therapeutic agents in clinical cancer trials and can be used as general guidelines for assessing therapeutic response whether or not a patient is enrolled in a trial. The most commonly used criteria, such as the Response Evaluation Criteria In Solid Tumors (RECIST), are based on the physical measurement of metastatic lesions. Bone metastases are often located in the axial skeleton and the irregular shape of bones such as the vertebra and pelvis make physical measurement difficult. In addition, bony metastases do not always respond to therapy with a significant change in size. Therefore, these traditional methods of evaluating therapeutic response are not generally applicable to bony metastases unless a measurable soft tissue mass extends from the cortex.62 In response to the need for criteria that can be used to evaluate bony metastases, a bone-specific set of criteria were developed at the M. D. Anderson Cancer Center (MDA criteria).3
The MDA criteria include quantitative and qualitative assessments of the behavior of bone metastases (Table 33-1). Response is divided into four categories: complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD). Quantitatively, these criteria define PR as a decrease of 50% or more in the sum of the perpendicular measurements of a lesion and PD as an increase of 25% or more in this sum. The response categories are defined as follows:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree