Chapter 14 Esophageal Cancer
Introduction
Esophageal cancer is a devastating diagnosis with a high morbidity and mortality. The overall 5-year survival for all stages combined remains dismally low at approximately 17%.1 Although it is a relatively uncommon malignancy, accounting for less than 1% of all malignancies, it has been steadily increasing in incidence since the 1980s, with an estimated 16,470 new cases and an estimated 14,530 deaths in the United States in 2009.2 Up to 38% of patients with esophageal cancer present late with locally advanced or metastatic disease and are not suitable for curative resection.3 However, there have been significant improvements in both staging and therapeutic options in recent years, leading to a greater understanding of the biology of the disease. Optimal management depends on effective multidisciplinary care that combines multimodality staging and combined modality treatment incorporating chemotherapy, radiation therapy, and surgery. For the radiologist, appropriate imaging requires a full understanding of the behavior and staging of esophageal cancer in addition to a familiarity with the treatment options available. For the surgeon and clinician, recognition of the limitations and pitfalls of each imaging modality is important in order to appropriately utilize the information obtained from imaging.
Epidemiology and Risk Factors
Esophageal cancer is a worldwide disease with an incidence that varies according to geographic location. The most endemic areas include Asia, southern and eastern Africa, northern France, and Great Britain.4 In the United States, esophageal cancer accounts for less than 1% of all cancers, but it has a high mortality and is responsible for 3% of all cancer deaths.2 The risk of esophageal cancer rises with age, with a median age at diagnosis of approximately 60 years. Males have a more than threefold risk compared with females.2
The two most common histologic types of esophageal cancer are squamous cell carcinoma (SCC) and adenocarcinoma (AC). Historically, SCC has been the more common of the two types; however, since the early 1980s, there has been a steady decline in the incidence of SCC and an even greater increase in the incidence of AC, particularly in white males.4
Risk factors for the development of SCC of the esophagus include alcohol intake, smoking, and lower socioeconomic status.5,6 Risk factors for AC of the esophagus include Barrett’s esophagus (epithelial metaplasia related to chronic gastroesophageal reflux), smoking, and increased body mass index; alcohol does not appear to be a significant risk factor for AC. Protective factors include high intakes of fresh fruit and vegetables and antioxidants and use of aspirin and other nonsteroidal anti-inflammatory drugs.7–9
An explanation for the rising incidence of AC of the esophagus and its striking male predominance (7:1) is unclear. Epidemiologic data do not support direct correlations with the increased incidence of gastroesophageal reflux, the more widespread use of lower esophageal sphincter–relaxing medications, the greater incidence of obesity, or the decreased incidence of Helicobacter pylori infection.10 Tobacco use among males has decreased in recent years, which may partly account for the decreasing incidence in SCC of the esophagus but does not explain the increasing incidence of AC. Screening those at increased risk of esophageal carcinoma is possible through the identification of certain risk factors (based on age, body mass index, and reflux symptomatology) but would consume considerable healthcare resources and is currently not justifiable on a population basis. However, in view of the increasing incidence of this disease and its high morbidity and mortality, the issue of surveillance may be revisited in the future.
Anatomy and Pathology
SCC and AC are both epithelial in origin but otherwise differ greatly in anatomic proclivity, risk factors, pathologic behavior and prognosis, and for staging and clinical purposes, they can be considered as separate diseases. SCC is a disease of nicotine and alcohol abuse, risk factors that affect the entire esophagus and partly explain why SCC arises in the mid- and upper thoracic esophagus above the level of the carina in up to 65% of cases.11 AC, conversely, is strongly linked to gastroesophageal reflux disease and Barrett’s esophagus and is almost always located below the level of the carina.12 SCC of the esophagus is also associated with a significantly worse prognosis than is AC.13
Because the behavior and surgical options for esophageal cancer vary according to its location, the esophagus is divided into four distinct anatomic regions14: the cervical esophagus extends from the cricopharyngeus muscle to the suprasternal notch; the upper thoracic esophagus extends from the suprasternal notch to the lower border of the azygos vein; the midthoracic esophagus extends from the lower border of the azygos vein to the inferior pulmonary veins; and the lower thoracic esophagus extends from the inferior pulmonary veins to the stomach and includes a variable portion of the intra-abdominal esophagus and the gastroesophageal junction.15 Gastroesophageal junction tumors have been further subdivided into types I, II, and III according to the relative extent of involvement of the esophagus or gastric fundus.16 According to the American Joint Committee on Cancer (AJCC) staging classification, Siewert type III tumors (those with an epicenter within 5 cm of the gastroesophageal junction but with proximal extension into the gastroesophageal junction or lower thoracic esophagus) are considered as primary esophageal tumors, and more distal tumors are staged as gastric carcinomas.
Histologically, the esophageal wall comprises the mucosa, the submucosa, the muscularis propria, and the adventitia. Local tumor invasion is determined according to the involvement of each of these histologic layers (Figure 14-1). There is no serosa surrounding the esophagus, facilitating local tumor invasion into pleura, pericardium, diaphragm, or peritoneum.
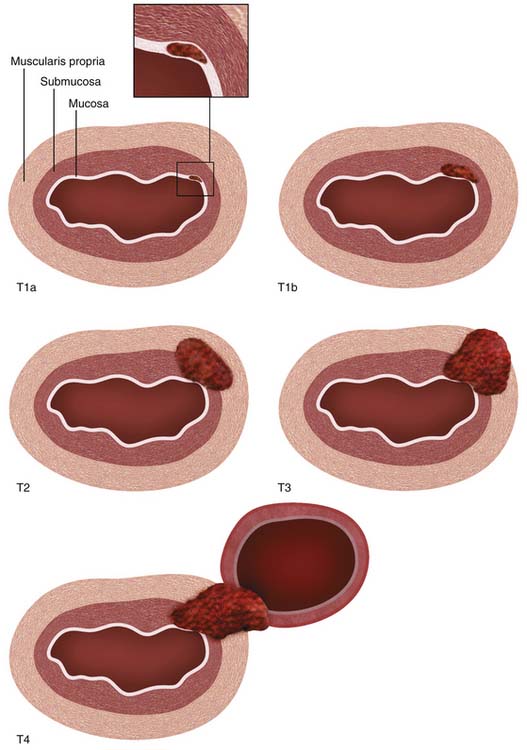
Figure 14-1 Schematic illustration of T descriptor of esophageal cancer. See Table 14-1 for comprehensive description.
A rich lymphatic network drains the esophagus with channels running both radially and longitudinally, facilitating early and distant dissemination of lymphatic metastases. Most of the lymphatics are concentrated in the submucosa, but they are also present in the lamina propria of the mucosa and connect to periesophageal lymph node stations and with the thoracic duct.17 The longitudinal lymphatic plexus in the submucosa facilitates orthogonal drainage both cranially and caudally, with the result that lymph node metastases may be located distant to the primary tumor without involvement of adjacent lymph nodes (“skip” metastases).
Locoregional lymph nodes are defined as those lymph nodes that are located close to the esophagus and that would commonly be resected at the time of surgery (Figure 14-2). These include lower cervical and supraclavicular lymph nodes; intrathoracic lymph nodes in the periesophageal region, mediastinum, and hilar regions; diaphragmatic lymph nodes adjacent to the crura; left gastric and paracardial lymph nodes; and common hepatic, splenic, and celiac lymph nodes.15
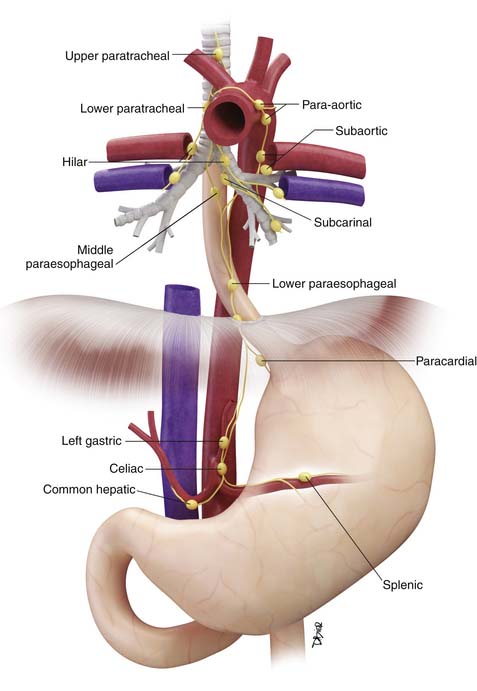
Figure 14-2 Schematic illustration of locoregional lymph nodes in esophageal cancer. See text for further discussion.
Key Points Anatomy and pathology
• SCC of the esophagus is more commonly located above the level of the carina and associated with a significantly worse prognosis than AC.
• Esophagus does not have a serosa, thereby facilitating adjacent organ invasion by tumor.
• Extensive submucosal lymphatic plexus facilitates both orthogonal and radial spread of metastatic disease in the lymphatics.
Clinical Presentation
Most esophageal carinomas, particularly AC, arise in the distal esophagus or gastroesophageal junction.18 Symptoms are often initially silent or nonspecific and are most often attributed to underlying chronic gastroesophageal reflux, manifesting as chronic or recurrent dyspepsia. More specific symptoms and signs, when they occur, typically present late, most often with weight loss, dysphagia, or odynophagia, by which time the primary tumor is locally advanced.
Tumors in the more proximal cervical esophagus may also present with dysphagia or signs of local tumor extension such as dysphonia or sympathetic nerve dysfunction.
By the time of presentation, distant metastases may already be present in up to 20% to 30% of patients and are often occult on conventional imaging.3,19 Advanced weight loss is a poor prognostic sign and nutritional replacement is an important facet of treatment.
Staging Evaluation
The most commonly used international staging system for esophageal cancer is the tumor-node-metastasis-grade (TNMG) classification published by the AJCC, currently in its seventh edition.15 According to this system, tumor descriptors are assigned based on the extent of local tumor invasion (T), nodal involvement (N), presence of distant metastases (M), and histologic grade (G) (Table 14-1). Stages are formulated according to combinations of descriptors that are associated with similar prognoses and are intended as a guide to management and prognosis (Tables 14-2 and 14-3).
Table 14-1 Tumor-Node-Metastasis Descriptors
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1a | Tumor invades lamina propria or muscularis mucosae |
| T1b | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades adventitia |
| T4a | Resectable tumor invading pleura, pericardium, or diaphragm |
| T4b | Unresectable tumor invading other adjacent structures such as aorta, vertebral body, or trachea |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in one or two regional lymph nodes |
| N2 | Metastasis in three to six regional lymph nodes |
| N3 | Metastasis in seven or more regional lymph nodes |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Histologic Grade (G) | |
| GX | Grade cannot be assessed—stage grouping as G1 |
| G1 | Well differentiated |
| G2 | Moderately differentiated |
| G3 | Poorly differentiated |
| G4 | Undifferentiated—stage grouping as G3 squamous |
The seventh edition of the AJCC staging classification incorporates several important revisions compared with the sixth edition. Most importantly, different staging classifications are used depending on whether the primary tumor is SCC (see Table 14-2) or AC (see Table 14-3), based on worldwide evidence that histopathologic type has an influence on prognosis (SCC in general having a worse prognosis than AC).13 However, the TNMG descriptors remain the same for both histopathologic types.
The T descriptor ranges from Tis (carcinoma in situ) to T4 (tumor invades into adjacent structures) (see Figure 14-1). New since the sixth edition of the AJCC staging system, T1 is now subdivided into T1a and T1b, depending on whether tumors are confined to the mucosa (T1a) or whether there is invasion of the submucosa (T1b). Also new since the previous edition, T4 is now subdivided into T4a (potentially resectable invasion of the pleura, pericardium, or diaphragm) and T4b (unresectable invasion into other adjacent structures such as the aorta and vertebral bodies). According to the National Comprehensive Cancer Network (NCCN) clinical practice guidelines,20 T1a or T1b tumors without nodal metastases are amenable to surgical monotherapy (endoscopic resection or esophagectomy) but more advanced tumors will require combined modality treatment involving chemotherapy, radiotherapy, and surgery. T4b tumors are unresectable.
The N descriptor has been simplified since the sixth edition with removal of the M1a descriptor (nonregional lymph node involvement). Celiac and supraclavicular lymph node metastases are now considered as locoregional lymph node metastases (rather than as M1a disease as in the sixth edition) (see Figure 14-2). The number of involved lymph nodes has been shown to have an important influence on survival and this is reflected in the subdivision of the N descriptor into N1 (1 or 2 lymph nodes involved), N2 (3-6 lymph nodes involved), and N3 (>6 lymph nodes involved). This subdivision also implies that an adequate lymph node dissection must be performed to ensure adequate pathologic staging and also to optimize survival. A recommended number of lymph nodes that should be removed has not been universally determined, but most would agree that at least 10 lymph nodes should be removed for early-stage carcinomas, and more (20-30 lymph nodes) for advanced tumors.21–26
Nonregional lymph node metastases and distant visceral metastases are both designated an M descriptor (previously subdivided into M1a and M1b) and are associated with a uniformly poor prognosis, with a 5-year survival of only 3%.1
Key Points Staging
• Different staging systems for SCC and AC.
• T1 descriptor subdivided into T1a (tumor confined to mucosa) and T1b (extension of tumor into submucosa).
• T4 descriptor subdivided into T4a (limited invasion of pleura, pericardium, or diaphragm) and T4b (invasion into other adjacent organs).
• N descriptor subdivided into N1 (1-2 lymph node metastases), N2 (3-6 lymph node metastases) and N3 (>6 lymph node metastases).
• M1 descriptor includes both nonregional lymph node metastases and distant visceral metastases.
Patterns of Tumor Spread
Local Extension
Most esophageal carcinomas are epithelial in origin (i.e., SCC and AC) and normally progress in a radial fashion from involvement of the mucosa, to breach of the muscularis mucosae with extension into the submucosa, to invasion of the muscularis propria and involvement of adjacent organs. The absence of a serosa means that, once tumor has invaded through the muscularis propria and adventitia, local organ involvement readily occurs. Once tumor has invaded into the submucosa, it can also spread longitudinally by several centimeters before involving the muscularis propria. Endoscopic ultrasound is the most accurate modality for assessing the depth of tumor invasion; however, even by this means, it is difficult to confidently identify tumors that are limited to the mucosa or to diagnose local organ invasion.27
Lymphatic
Lymph node metastases spread radially to adjacent periesophageal lymph nodes and longitudinally via the submucosal lymphatic plexus and via the thoracic duct to more distant lymph node stations. Longitudinal lymphatic spread is in a roughly predictable fashion: tumors located in the cervical and upper thoracic esophagus drain preferentially in a cranial direction to cervical lymph nodes; tumors in the distal esophagus and gastroesophageal junction drain caudally to intra-abdominal lymph nodes; and tumors in the midthoracic esophagus may drain in either direction.28 However, lymphatic spread is not limited to these trends; lymph node metastases along the recurrent laryngeal nerves in the neck can still occur with distal esophageal tumors.28,29 Furthermore, because of the rich submucosal lymphatic plexus, “skip” metastases to distant lymph node stations may occur while bypassing locoregional lymph nodes; such skip metastases are found in 10% to 20% of resected tumors, and in surgery performed with a curative intent, these are an important argument for extended lymph node dissection.27,29
Although lymphatic spread of esophageal cancer occurs early, the probability of lymph node metastases increases with greater local tumor invasion. Lymph node metastases occur in up to 33% of patients with early intramucosal tumors, 67% of intramural tumors, and 89% of tumors that have invaded through the esophageal muscularis propria.27,30
Hematogenous
Hematogenous metastases to distant organ sites are common and are estimated to be present in up to 18% to 30% of patients at the time of presentation.3,31 The risk of hematogenous spread of tumor increases with more advanced local tumor invasion and nodal involvement, but can also occur early with small primary tumors and no apparent nodal metastases. The most common sites of distant visceral metastases include the liver, bones, lungs, and adrenal glands, but they may also occur in more unusual sites (brain, skeletal muscle, subcutaneous fat, and thyroid gland) and may be occult by CT imaging.32 The detection of distant metastases constitutes incurable disease and is associated with a very poor prognosis.
Peritoneal
Locally advanced tumors of the intra-abdominal esophagus or gastroesophageal junction can spill tumor cells directly into the peritoneal cavity and seed distant intra-abdominal or intrapelvic sites. Peritoneal metastatic disease constitutes a contraindication to surgery but is difficult to diagnose prospectively by imaging owing to the typically small volume of disease and its occult nature on cross-sectional images. In general, the diagnosis is made either at the time of surgery or preoperatively by laparoscopic exploration.33 The peritoneum represents an important source of recurrent disease after definitive therapy.
Key Points Tumor spread
• Lymphatic and hematogenous metastases occur early and their probability increases with locally advanced tumor.
• “Skip” nodal metastases occur in 10% to 20% of resected tumors.
• Hematogenous metastases are present in up to 30% of patients at the time of initial presentation.
• Hematogenous and peritoneal metastases may be occult on CT imaging.
Imaging
EGD is critical for initial diagnosis and EUS is an extremely useful tool for nodal evaluation in specialized centers, but a full discussion of these endoscopic techniques is beyond the scope of this chapter. Barium studies are important for evaluating the length and severity of stricture, which can be used to assess the need for stent placement and for guiding radiation therapy planning, but do not provide useful staging information. Standard radiologic baseline staging is based on contrast-enhanced CT and, for patients with potentially resectable tumors, PET/CT (Table 14-4).
PET/CT imaging with 2[18F] fluoro-2-deoxy-D-glucose (18F-FDG) has greatly changed the way in which esophageal cancer is managed and should now be considered an indispensable imaging modality for baseline staging and therapeutic response evaluation. It provides both functional and anatomic information of great use for guiding clinical decision-making; however, its optimal utilization requires an understanding of its limitations and the interpretative pitfalls that may be encountered. Because conventional contrast-enhanced CT (Table 14-5) provides better-quality images of the soft tissues and particularly of the lungs, both CT and PET/CT are complementary modalities for the evaluation of esophageal cancer.
Table 14-5 CT Imaging Parameters
| PARAMETER | |
|---|---|
| Volume of interest | Midcervical neck to symphysis pubis |
| kVp | 100-140 |
| Oral contrast medium | 500-1000 mL dilute contrast medium, 45 min before imaging |
| Intravenous contrast medium | 300-350 mg/dL nonionic iodinated contrast |
| Injection technique | 100-120 mL at 3-5 mL/sec |
| Scan phase (time from end of injection to start of scan [in sec]) | Thorax: phase of peak pulmonary arterial enhancement (20-25 sec) Abdomen/pelvis: portal venous phase (50-60 sec) |
| Image re-formats | 2-3-mm-thin axial slices in soft tissue (center, 50 HU; width, 350 HU) and lung (center, 600 HU; width, 1600 HU) windows with appropriate vendor-specified soft tissue and lung/bone reconstruction algorithms |
| Optional additional multiplanar re-formats | Coronal and sagittal oblique |
Primary Tumor
Computed Tomography
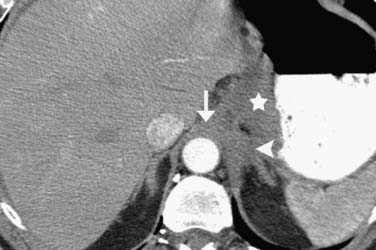
On axial CT scans, the primary tumor may be visualized as a focal or diffuse area of esophageal wall thickening (Figure 14-3). An esophageal wall thickness greater than 5 mm has been described as abnormal34; however, in practice, it is difficult to accurately measure the esophageal wall thickness because it is frequently nondistended and often cannot be accurately differentiated from adjacent structures such as the thoracic duct and small periesophageal lymph nodes. Compared to EGD/EUS, CT has a relatively poor sensitivity for depiction of the primary mass (~67%) and cannot resolve invasion of the different histologic layers of the esophageal wall.35
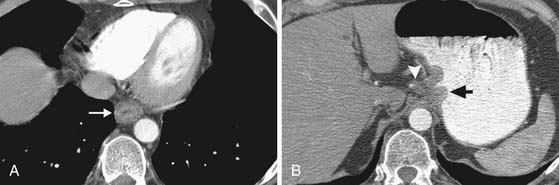
Invasion into adjacent structures (T4 disease) may be suggested on CT by contiguity between the esophagus and the adjacent organs and loss of the normal periesophageal fat planes. Aortic invasion is suggested by encasement greater than 90 degrees and diaphragmatic invasion by loss of the retrocrural fat planes (Figure 14-4). Tracheal invasion may be suspected where a midesophageal tumor bulges into the posterior membranous portion of the tracheal wall (which normally has a straight or convex shape). In practice, however, the fat planes are often absent without invasive disease, and periesophageal fat stranding may also occur secondary to fibrosis or inflammation incited by the primary tumor. In the absence of evidence of frank invasion into surrounding structures, a T4 status is often impossible to determine with certainty by any modality and is a diagnosis most often made at the time of attempted resection.
Positron-Emission Tomography/Computed Tomography
Most, but not all, esophageal malignancies are FDG-avid. Although there is some evidence to suggest that the metabolic activity of the primary tumor (as measured by the maximum standard uptake value [SUVmax] or metabolic tumor volume [MTV]) is of prognostic value, there are conflicting reports as to the reliability of this correlation.36–39 Although an understanding of the relationship between FDG uptake and tumor biology is still evolving, the baseline metabolic activity of esophageal cancer has not yet been incorporated into routine clinical decision-making or staging.
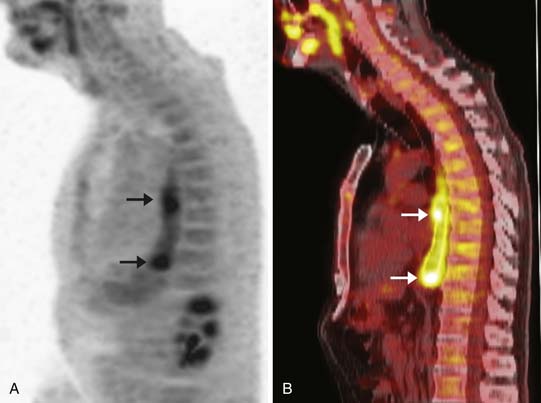
In patients with esophageal cancer, FDG uptake in the esophagus is most often secondary to activity in the primary tumor itself but can also occur secondary to multiple additional factors that may confound the apparent longitudinal extent of the tumor. Esophagitis or mucosal ulceration secondary to acid reflux is a common cause of false-positive FDG uptake, appearing as linear or focal areas of high activity in the distal esophagus. After endoscopic esophageal stent placement, there is often intense surrounding FDG uptake that cannot be differentiated from the primary tumor itself (Figure 14-5). Another frequent cause of false-positive FDG uptake in the esophagus is inflammation after endoscopic biopsy of the mucosa. For all of these reasons, evaluation of the apparent metabolic activity of the primary esophageal tumor should be correlated with the recent clinical history and findings at endoscopy.