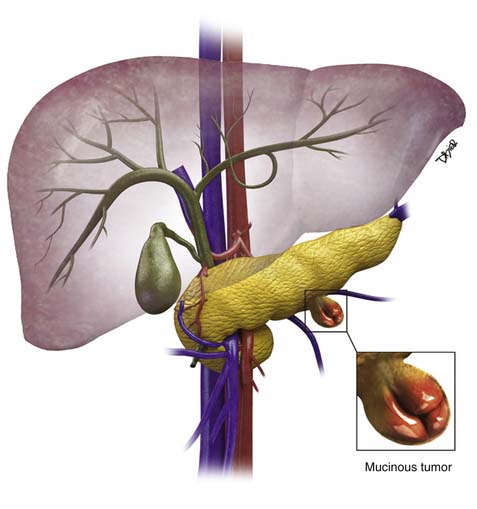
Chapter 12 Cystic Pancreatic Lesions
Introduction
Cystic lesions of the pancreas include malignant and benign processes and may or may not cause clinical symptoms. Most of these lesions are seen incidentally on cross-sectional imaging and can be of various sizes. The risk of malignancy in a symptomatic cyst can be high, whereas asymptomatic cysts can be benign, malignant, or premalignant. Pancreatic cystic neoplasms account for 10% to 15% of pancreatic cysts, less than 1% of primary pancreatic malignancies.1
Currently, computed tomography (CT) and magnetic resonance imaging (MRI) are used in assessment of pancreatic cystic lesions. CT is more widely available because it requires less scan time and is the most accessible and, hence, is more commonly used. MRI is able to identify communication of the cystic lesion with the main pancreatic duct, although with currently available thin-section imaging, CT has a similar ability.2 Fluoro-2-deoxy-D-glucose (FDG)–positron-emission tomography (PET)/CT has been used to differentiate a malignant from a benign cystic lesion depending on its metabolic activity.3
CECT and MRI is not operator-dependent and can visualize the entire pancreas without difficulty. Endoscopic ultrasound (EUS) is operator- and patient-dependent, and EUS-guided fine-needle aspiration (FNA) has a complementary role in this difficult task by providing additional real-time assessment and cystic content for further analysis. Equipped with a Doppler device, it allows tissue sampling by FNA avoiding the major vessels. PET/CT is an evolving modality for detecting malignancy in cystic pancreatic lesions. It has been shown to detect invasive cancer in a cystic lesion; however, it cannot identify carcinoma in situ.3 This modality can help detect distant metastases in the setting of invasive cancer.4 Pancreatic cystic lesions may be identified at transabdominal US. However, it is limited in patients with large body habitus, and overlying bowel gas may obscure the lesions. Calcifications within the lesion are better seen on the CECT; septations may or may not be well seen depending on the size of the lesion and thickness of the septations. MRI is able to identify septations better as well as the contents of the cyst fluid if they are hemorrhagic; however, detection of mucin is equivocal. Even incidentally found cystic lesions of the pancreas should undergo diagnostic evaluation regardless of size. EUS can be used to evaluate the cyst, and concomitant cytologic analysis of cyst fluid can be obtained by EUS-FNA. The pancreatic cyst fluid analysis should include staining for mucin and measurement of cyst fluid amylase, lipase, and various tumor markers such as carcinoembryonic antigen (CEA).
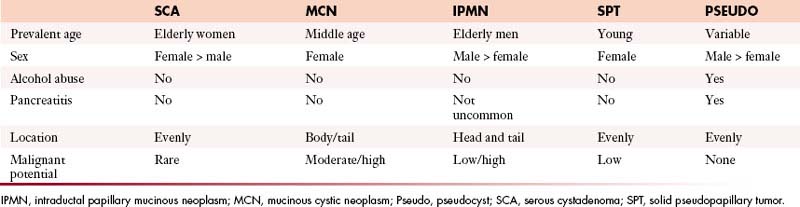
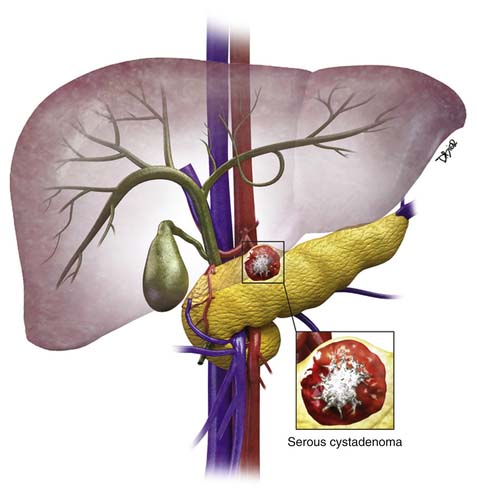
Pseudocysts make up the majority of all cystic lesions of the pancreas, the rest comprise cystic tumors and true cysts. Hydatid cysts of the pancreas are rare and should be considered as a differential diagnosis of a cystic pancreatic lesion in countries where this disease is endemic.5 Pancreatic primary cystic tumors fall into one of three major groups: serous tumors (including SCA and cystadenocarcinoma), mucinous tumors (including mucinous cystadenomas, mucinous cystadenocarcinomas, intraductal papillary adenomas, and intraductal papillary adenocarcinoma) and solid pseudopapillary tumors (SPTs). The majority of cystic pancreatic tumors are asymptomatic and slow-growing. When the patients are symptomatic, the symptoms are caused by mass effect that is usually vague and poorly localized. Intraductal papillary mucinous tumors (IPMTs) may present with pain that may mimic chronic pancreatitis.6 On cross-sectional imaging, they can be characterized as simple, containing no internal nodularity, or complex, containing internal septations or mural nodules. They can also be classified as unilocular and multilocular depending on the number of locules present.
I Pancreatic Pseudocysts
Epidemiology and Risk Factors
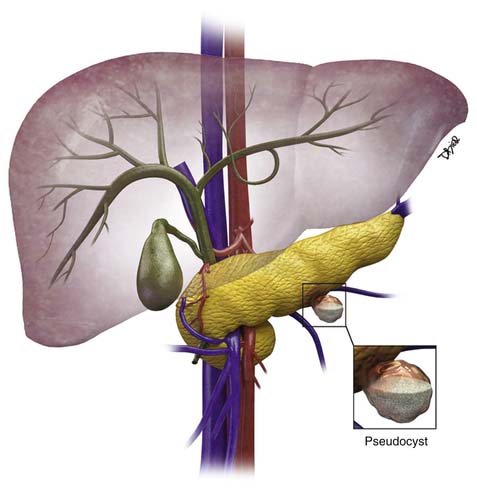
The majority of cystic lesions found in the pancreas are pseudocysts and they account for 70% of all cystic lesions.7 They lack an epithelial lining and have a fibrous capsule. Pancreatic pseudocysts occur after an episode of pancreatitis and are caused by leakage of pancreatic enzymes, such as amylase and lipase, which cause fat necrosis and hemorrhage owing to erosion of adjacent vessels.
Clinical Presentation
Patients with pancreatitis may present with nausea, vomiting, abdominal pain, or sepsis due to infection or obstructive jaundice.8 Because there is leakage of pancreatic enzymes, fistulation into nearby structures, such as the common bile duct, esophagus,9,10 and pleura,11 can occur. The pancreatic pseudocysts seldom demonstrate internal hemorrhage, however this finding is associated with increased mortality. The pseudocysts can erode the adjacent vessels and cause pseudoaneurysms, which may rupture, leading to hemorrhage. The most common arteries involved are the splenic, gastroduodenal, and superior pancreaticoduodenal.12 Pseudocysts are commonly seen in males and usually are seen in alcoholics or can be associated with abdominal trauma.13
Imaging
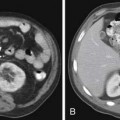
Pseudocysts (Figures 12-1 and 12-2) on cross-sectional imaging usually appear as unilocular lesions, without internal septations or mural nodules. They do communicate with the main pancreatic duct.7 These cysts on imaging have an irregular wall in the early stages, but tend to evolve and become well marginated.14 On CECT, pseudocysts look like a fluid collection, which may either have an imperceptible wall or a thick wall that may or may not show enhancement since it is made of fibrous tissue.14 On MRI, pseudocysts may have a high signal on T1-weighted imaging (T1WI) due to blood products. On magnetic resonance cholangiopancreatography (MRCP), they may show communication with the main pancreatic duct. On US, they may appear as thin-walled unilocular anechoic masses with increased through transmission. Complicated cysts may have low-level internal echoes with a fluid debris level or can have internal septations. If calcification is present within the wall of the cyst, it may obscure the cyst. They do not have enhancing nodules, and if present, one must consider a mucinous cystic neoplasm (MCN) in the differential. One clue that can be seen on CT or MRI is peripancreatic stranding, which is considered the hallmark of pancreatitis and, when associated with a cystic lesion, is suggestive of a pseudocyst.
II Simple Cysts
Epidemiology and Risk Factor
True cystic lesions of the pancreas are common,4,15 are lined by epithelium, and can be seen in patients with von Hippel–Lindau (vHL) disease,16 cystic fibrosis, and polycystic kidney disease. These cysts have a similar imaging appearance to the simple cysts seen in the liver and the kidneys.15
Imaging
On CT, they have Hounsfield unit (HU) values of fluid. On MRI, they have a high signal on the T2-weighted imaging (T2WI) and a low signal on T1WI and do not show enhancement. They do not have mural nodules. On US, they are anechoic with posterior acoustic enhancement. The differential of these cysts includes MCN because there is no distinguishing radiologic finding between the two. When these cysts are larger than 3 cm, EUS can be performed and a biopsy can be obtained to determine the cystic contents and exclude a mucinous neoplasm; these cysts are not usually resected.
III Mucinous Non-neoplastic Cyst
Epidemiology and Risk Factors
Mucinous non-neoplastic cysts (MNCs) are common cystic tumors of the pancreas first described in 2002 by Kosmahl and coworkers.17 The cause and the development of MNCs are not known. It may be speculated that they are caused by a developmental defect of the pancreas, resulting in a focal cystic transformation of the duct system.
Pathology
MNCs are lined by tall columnar epithelium, which secretes mucin.18 Their maximum diameter ranges from 3 to 12 cm, they may be either unilocular or multilocular, and they may be filled with turbid or bloody fluid.17 These tumors do not have an ovarian stroma, which is a characteristic of mucinous cystadenomas. These cysts also do not communicate with the main pancreatic duct, do not show cellularity or have papillary projections as seen in IPMNs, and are believed to be benign.17
Imaging
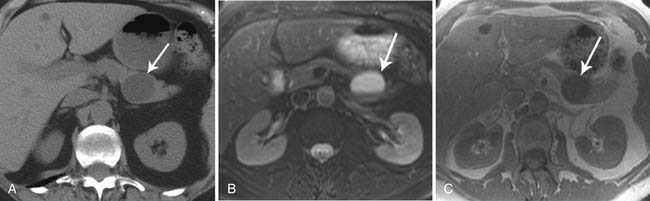
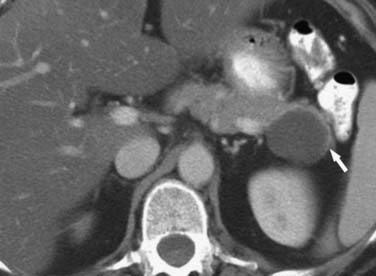
On imaging, MNCs (Figure 12-3) are usually small and unilocular, with thin septa. On MRI, they have a signal intensity of simple fluid; on CECT, their HU is less than 20. On imaging, the appearance of MNCs cannot be easily differentiated from that of mucinous cystadenomas, especially if the mucinous cyst is large or has a thick wall. One of the important distinguishing factors is that the mucinous cystadenomas are commonly seen in females, whereas the MNCs have no gender predilection.19 If these cysts are larger than 2 cm, EUS-FNA can be performed and the cyst contents can be differentiated from those of other mucinous cystic neoplasms on immunohistochemical staining (Table 12-1).
Treatment
Usually MCNs do not require treatment unless they become symptomatic owing to internal hemorrhage or cause biliary obstruction, which is rare.17
Surveillance
If present in the head of the pancreas, they can cause ductal obstruction and can be followed at different intervals depending on size.
Anatomy and Pathology
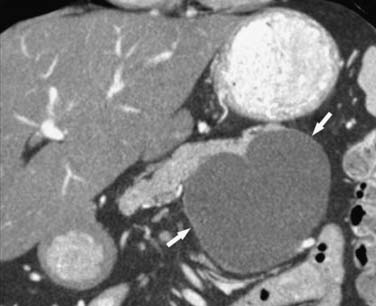
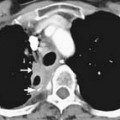
MCNs (Figure 12-4) are generally found in the body and tail of the pancreas, they are usually solitary, and their size ranges from 6 to 35 cm.20 They are lined with an inner layer of cells that secrete mucin and an outer layer of cells that resemble the ovarian stroma.4,21 They have several locules and a thick wall.6 These tumors do not communicate with the main pancreatic duct unless they erode into the duct, causing a fistula.6,22 The number of locules are fewer than six and the size of the locules is generally greater than 2 cm. They may contain watery fluid or hemorrhagic, necrotic, or mucinous material. These tumors usually have intramural nodules, which histologically may range from high-grade dysplasia to invasive carcinoma.22 MCNs resemble the MCNs of the ovary and are exclusively seen in women of reproductive age (>95%) with a mean age of 45 years, and are typically present in the body or tail of the pancreas.19,20,22,23 Curvilinear calcifications may be noted in the periphery of the tumor and/or capsule in 10% to 25% of cases.14
Clinical Presentation
They may present with vague abdominal discomfort and have symptoms such as weight loss and anorexia.
Imaging
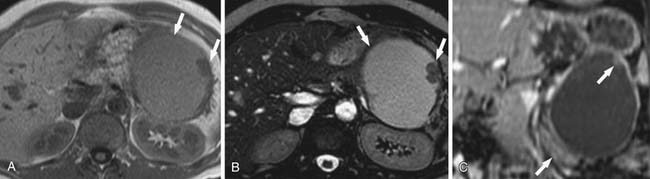
On cross-sectional imaging, these tumors may appear as a single large cyst or a large cyst with multiple locules with a thick outer wall, thick septations, and enhancing intramural nodules. Sometimes, calcification of the outer wall can be seen6; the outer wall of the cyst may enhance on the delayed phase, a finding that correlates with a fibrotic capsule seen on histopathology. On MRI, the fluid within the cyst may have a low signal on the T1WI and a high signal on the T2WI. However, increased signal on the T1WI has been observed owing to presence of mucin,24 but unfortunately, this is unreliable. The presence of enhancing nodules should raise the suspicion for malignancy.23
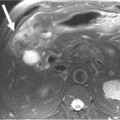
On ultrasound, MCNs (Figure 12-5) can appear as well-demarcated, anechoic, thick-walled cystic masses and may have echogenic thick septa, internal nodularity, or mural calcification. Differential diagnosis of these lesions include a pseudocyst; however, they can be differentiated on serial follow-up imaging in which the pseudocyst may decrease in size whereas the mucinous neoplasm usually persists.
Anatomy and Pathology
These masses are usually seen in the body and the tail of the pancreas. The patients with invasive mucinous carcinoma are older than those with noninvasive mucinous cystadenoma; this finding is suggestive of a progression from cystadenoma to cystadenocarcinoma.20 The earliest genetic change seen within MCNs is the mutation of the KRAS2 oncogene located on chromosome 12p25 and is present in 90% of the MCNs with carcinoma in situ.25 MCNs have a progression toward malignancy that is similar to that of pancreatic intraepithelial neoplasia.25
Clinical Presentation
These may present with vague abdominal pain and discomfort and, rarely, can cause pancreatitis.
Imaging
Although the presence of enhancing nodules and septations correlates with malignancy, absence of these findings does not preclude malignancy. On imaging, mucinous cystadenocarcinomas (Figure 12-6) are seen as large, complex, cystic pancreatic masses that can be distinguished from mucinous cystadenomas by the presence of intracystic enhancing soft tissue. These tumors are usually large (>6 cm), and malignancy is usually not seen in tumors smaller than 4 cm unless intramural nodules are present.20 Hence, any enhancing soft tissue within a cystic neoplasm depicted on CECT or MRI is considered an indication for resection; however, all mucinous lesions are considered premalignant and are generally resected because, radiologically in the majority of cases, discrimination between benign and malignant MCN is currently impossible. We have usually seen that, when the adjacent vessel such as the splenic vein is occluded, this finding may suggest a trend toward malignancy. Staging is similar to that of pancreatic adenocarcinoma.
Treatment
Surgical resection is recommended for all patients with suspected MCN who are suitable operative candidates. Whereas surgical resection of patients with noninvasive disease is usually curative, invasive MCN is characterized by frequent recurrence and consequently poorer survival; preoperative or postoperative chemoradiation may be useful in these patients.26,27 Gemcitabine appears to be effective in the case of pancreatic cystadenocarcinomas with peritoneal metastases.28,29 This treatment strategy is commonly offered to patients with resectable pancreatic cancer in order to achieve margin-negative resection and to restrict aggressive surgical therapy for those patients whose cancers have a favorable biology (or those cancers that do not progress during the preoperative chemotherapy phase).30
Surveillance
Key Points Mucinous cystic neoplasms
• Mucinous cystadenomas and carcinomas have ovarian stroma.
• They are unilocular and may have papillary projections.
• They commonly do not communicate with the main pancreatic duct.
• They are exclusively seen in menstruating females.
• On EUS-FNA, the CEA can be high as 200 to 500 and the amylase is low.
VI Intraductal Papillary Mucinous Tumors
Introduction
The three main types of IPMTs are classified based on their location: that is, the main duct type, the side branch duct type (BDIPMT), and the combined type.31 These tumors produce mucin, which accumulates in the ducts and results in cystic dilatation of the duct. Most commonly, IPMTs involve the main pancreatic duct, but they may also affect the side branches.32 Depending on their size and type, they can be premalignant or frankly malignant. They may exhibit a spectrum of dysplasia ranging from minimal mucinous hyperplasia to invasive carcinoma.33 The differential diagnosis of BDIPMTs may include a pseudocyst.
Epidemiology and Risk Factors
IPMTs were first described by Ohashi and colleagues in 1982, and the term was conceived by Sessa and associates in 1994.32 These tumors arise from the columnar epithelium of the pancreatic duct. The cells become dysplastic and form papillary projections that extend into the pancreatic ducts and secrete mucin. These tumors are more commonly seen in males, with a male-to-female ratio of 2:1. These patients typically present in the seventh to eighth decades of life. Initially, IPMNs were thought to represent only 5% of all cystic lesions, but they are being reported increasingly in numbers, likely owing to increased awareness of the disease and the use of cross-sectional imaging modalities. The older term for IPMN was “duct ectatic mucinous tumor.” This is a mucin-producing tumor that arises from the pancreatic ductal epithelium. According to the World Health Organization (WHO), IPMN is classified based on the degree of epithelial dysplasia: adenoma, borderline tumor, and carcinoma (either in situ or invasive).34
Anatomy and Pathology
IPMNs may be classified depending on disease process whether it involves the main pancreatic duct (Figure 12-7) or isolated side branches. They may be also characterized depending on whether they produce a diffuse pattern of ductal dilatation or a segmental cystic appearance.35 The location of the tumor is an important factor for the prognosis.36 Twenty percent to 30% are multifocal and 5% to 10% involve the entire pancreas.22,37