Fig. 2.1
Ablatherm® integrated imaging device
The lateral position of the treatment allows gas bubbles produced in the coupling liquid during heating of the prostate to rise by buoyancy to a position outside the field of view of the imaging probe, and therefore of the therapy transducer. The Ablatherm II® software includes four treatment protocols with specially designed treatment parameters depending on the clinical use: Primary care (standard), retreatment, radiation failure and post brachytherapy. The treatment plan involves setting up the probes to target the thermal lesion within the prostate. The operator defines the boundaries of the target area using the US scanner. The Ablatherm II® is then switched to treatment mode and the computer-driven module induces lesions using the HIFU transducer. To treat the entire target area, several sonications are made side-by-side, first moving the head following the transverse plane (right-left), and then following the longitudinal plane (perpendicularly to the transverse plane). The lesion height may be adjusted between 19 and 24 mm to match the size of the target area. The device offers real-time ultrasonic monitoring of the treatment because HIFU-induced lesions are visible using standard ultrasound as hyper-echoic areas, but their extent is not always accurately defined. However, at the end of the procedure contrast-enhanced ultrasound with Sonovue® (Bracco Imaging, Switzerland) may be implemented to validate treated volumes and to define areas for treatment completion.
2.3.2 Sonoblate 500
The Sonablate 500® (SonaCare Medical LLC, Charlotte, NC, USA) is based on a console, a fully integrated probe (Fig. 2.2) and a module for degassing and circulating chilled water in the probe tip. The console consists of a portable system with a display monitor, an articulating arm with reliable probe holding capabilities and a stepper motor with 3-axes of precise movement. The transrectal probe uses double-sided and dual-mode transducers for imaging (6.3 MHz) and treatment (4 MHz). The double-sided transducer is available with two focal lengths (30–40 mm) that move robotically to follow the precise treatment plan devised by the physician. The size of an elementary thermal lesion is 10–12 mm long and 3 mm in diameter. The treatment is performed in the supine position. The system uses a treatment protocol allowing adjustable power settings for customizable treatment by the physician. The treatment is carried out in two or three consecutive layers (based on anterior-posterior dimensions of the prostate), beginning with the anterior portion of the prostate and moving to the posterior part by changing the focal length during the procedure. The choice of the focal length depends on the size of the prostate. The maximum prostate size that can be treated is 40 mm AP dimension. The latest version of the device uses a pulse echo back-scattered ultrasound (RF signals) -processing algorithm called TCMTM (Tissue Change Monitoring) to check in real-time whether the treatment was sufficient. A pulse echo RF signal is sent to the treatment site prior to delivery of HIFU energy, and then the second signal is sent post HIFU delivery to the same treatment site. The TCMTM algorithm, in real time, calculates and quantifies the tissue change that takes place in the treatment site and displays the degree of HIFU lesion completeness on the screen. This feedback allows the physician to re-treat the sites that were not treated optimally. For more details on the Sonablate 500® see Chap. 6 of “Therapeutic Ultrasound: Mechanisms to Applications” (Tavakkoli and Sanghvi 2011).

Fig. 2.2
Sonoblate® device
2.3.3 Focal One
A new device, FocalOne® (EDAP TMS, Vaulx-en-Velin, France), has recently been developed by EDAP to deliver HIFU treatment by combining all the latest technologies in imaging and treatment for an ideal PCa therapy: Accurate and MRUS-based fusion imaging guidance, a non-invasive surgical approach, precise and efficient therapeutic energy and end-of-treatment validation imaging.
In contrast to Ablatherm II®, the FocalOne® does not use a special bed and consists of a single module (Fig. 2.3). The treatment sequence now includes a pre-treatment step in which MR prostate images of the patient are imported from PACS or CD and elastic fusion is automatically performed to match the 3D contours of the prostate on the MRI and ultrasound images. For the pre-treatment step, the 3D MR target is automatically displayed on live ultrasound, and the software makes it possible to design a precise target area with accuracy better than 1 mm. A second screen displays the conformational treatment with an ultrasound image of the prostate and the target area defined in the prostate. The software can be used to modify in real-time the shape and size of the treatment area; this was not possible with the Ablatherm II®. Finally, in post-treatment sessions, contrast-enhanced Ultrasound with Sonovue® is used to validate treated areas and to define the areas for treatment completion. In the days following the procedure, review of treatment images is available for comparison with MR follow-up images.


Fig. 2.3
Focal One® device
A new probe based on the technology of “dynamic focusing” (beam steering) has been developed to deliver localized thermal therapy to the areas of the prostate gland via the transrectal approach. The probe has the same ergonomic design and the same ultrasound imaging transducer as that of the Ablatherm II® (Fig. 2.3). The HIFU transducer consists of an annular array with 16 individual concentric rings. All of the rings have the same surface area and are fed through their own amplifier channel (16 channels). The geometric focal point of the firing head is located at 60 mm from the front face, instead of 40 mm for that of the Ablatherm II®. The annular array makes it possible to steer the ultrasound beam at different depths and to adjust the treatment according to the thickness of the prostatic tissues to be treated. In particular, it is possible to focus the ultrasound beam at a depth other than the natural radius of curvature of the transducer. The objective is to fit the treated volume as closely as possible to the geometry of the prostate, approaching a model of lesions formed “point by point” with short sub-sonications performed at different depths. For this, a phase shift is performed electronically on each ring to steer the HIFU beam along the axis of penetration, allowing enough depth to be reached to treat large prostates.
The advantage of this new probe is that it allows for a correction of the major disadvantages associated with the use of a fixed focus transducer. The 16 annular elements activated by a multichannel generator are used to split each sonication into sequences of one-second sub-sonications. Each sub-sonication ablates an ellipsoidal volume of 5 mm in height. The lesion consists of a small region of coagulation necrosis with reduced cavitation effects and concentrated thermal diffusion. The device makes it possible to add dynamic focusing sub-sonications along the axis of penetration of the ultrasound beam. It is therefore possible to obtain small necrotic lesions of 5 mm long with a shot of 1 s. Although the damage obtained with a single shot is small, large treatment zones can be obtained up to 40 mm in length, by adding 8 successive sub-sonications of 5 mm each. The fact that the height of the lesions can be varied between 5 and 40 mm allows for better matching of the treated area to the morphology of the prostate, as the focal point being always inside the prostate. The thermal dose delivered to the prostate is theoretically homogeneous, with fewer thermal diffusion phenomena, laterally towards the neurovascular bundles and vertically toward the striated sphincter. The anatomical distribution and homogeneous thermal dose throughout the prostate gland is expected to achieve better oncological results regardless of the size of the prostate. Furthermore, the absence of thermal diffusion should provide a significant reduction in side effects, such as incontinence and impotence. This new probe can be used to treat many, very small prostates with a focal point that will always be maintained within the gland, as well as large prostates with antero-posterior distances of up to 35 mm, which corresponds to prostates of about 50 cc. This is an improvement over the fixed focus transducer that could only treat prostates with a maximum antero-posterior distance of 26 mm, which corresponded to prostates with a maximum of 30 cc. Finally, it is possible to shorten the duration of treatment for a given treated volume. In fact, the shots fired at shallow depth will be associated with reduced firing power. Rest periods, with the aim of limiting the temperature rise in the rectal wall, could therefore be shortened.
2.3.4 MRgFUS Devices
Magnetic resonance guided focused ultrasound surgery (MRgFUS) was recently presented as a method for ablation with focused ultrasound under magnetic resonance imaging guidance. This approach has the advantage of improved targeting and real-time temperature monitoring. To date, two different approaches have been used for MRgFUS of the prostate: One with a transrectal probe compatible with the ExAblate® system (InSightec, Haifa, Israel) under a 1.5 T GE MRI, and another with a MRI-compatible ultrasound applicator to deliver controlled thermal therapy to the regions of the prostate gland via a trans-urethral approach (Profound Medical Inc., Toronto, Canada). The potential of both technologies is currently being demonstrated in Phase I clinical trials, but only a few studies have been conducted in therapy of PCa with human patients (Zini et al. 2012; Chopra et al. 2012), and most of the currently available literature on MRgFUS for prostate uses a canine model for research (Siddiqui et al. 2010; Chopra et al. 2009).
2.4 Long-Term Outcomes of HIFU in PCa Treatment
This section gives an extensive review of clinical outcomes in all uses of HIFU technology for treatment of localized PCa.
2.4.1 HIFU as Primary Care Treatment
The recommendations and updated guidelines on the use of HIFU for PCa as a primary treatment concern patients with localized PCa (clinical T1-T2 stage Nx/0 M0 PCa) for whom radical prostatectomies are not an option for one of the following reasons: Age >70 years old, life expectancy ≤10 years, major co-morbidities which preclude surgery or the simple refusal on the part of the patient to undergo surgery (Rebillard et al. 2003; AURO 2009). Among the publications on HIFU as a primary therapy for PCa, 16 studies report on a series of at least 50 patients (Uchida et al. 2006a, b, 2009; Crouzet et al. 2010a, b, c; Lee et al. 2007; Poissonier et al. 2007; Ahmed et al. 2009; Blana et al. 2008a, b, 2009; Mearini et al. 2009; Misrai et al. 2008; Ganzer et al. 2008; Turoff et al. 2003; Chaussy and Thuroff 2001; Gelet et al. 2000), while the others report on fewer patients (Ficarra et al. 2006; Challacombe et al. 2009; Maestroni et al. 2008; Koch et al. 2007). Follow-up varies significantly between series (range: 6 months to 6.4 years). In most cases, the PSA nadir was reached 3–4 months after treatment by HIFU, and was = 0.05 ng/mL in 55–91 % of the cases. Many studies have demonstrated that the PSA nadir was a significant predictor of HIFU failure. Patients with a PSA nadir over 0.5 ng/mL must be carefully monitored (Lee et al. 2007; Ganzer et al. 2008). A PSA nadir >0.2 ng/mL after HIFU has been associated with a four times greater risk of treatment failure, which is defined as a positive biopsy after HIFU treatment (Uchida et al. 2006a).
The 7-year disease-free survival rate in the longest follow-up multicenter studies was 75 %, 63 % and 62 % for low, intermediate and high-risk patients, respectively, and the 8-year cancer-specific survival rate was 99 % (Crouzet et al. 2010a, b, c). Complication rates are low with sloughing occurring in 0.3–8.6 %. Impotence occurs in 20–77 % of patients and bladder outlet obstruction in 12–22 %. The incontinence rates reported in a recent study were grade I (4–17.5 %) and grade II and III (0–5 %) (Chaussy et al. 2005; Crouzet et al. 2011). In our institution, we have recently reviewed the results of 880 patients. The mean age was 70 years old. Stratification according to d’Amico’s risk group was low, intermediate and high in 36 %, 48 % and 16 %, respectively. Median follow-up was 41 months. Median PSA nadir was 0.1 ng/mL. The overall and cancer-specific survival rates at 7 years were 90 and 98 %, respectively. The metastasis-free survival rate at 7 years was 96 %. The 5- and 7-year disease-free survival rates were 75–62 %, 59–50 % and 45–39 % for low, intermediate and high risk patients, respectively (p = 0.0001) (Crouzet et al. 2010a, b, c). Recent articles published in 2013 from three European urology departments confirm the long-term efficacy of HIFU treatment (Crouzet et al. 2014; Thüroff and Chaussy 2013; Ganzer et al. 2013).
In a study from a prospective database, Shoji et al. included 326 patients who filled in self-administered questionnaires on urinary function, QOL and sexual assessment (Shoji et al. 2010). The FACT G, FACT-prostate and IIEF 5 were used. Maximum flow rate and residual urine volume were significantly impaired at 6 months (p = 0.010) after HIFU, even though they returned to baseline values at 12 or 24 months after HIFU. The total FACT-G score significantly improved at 24 months (p = 0.027) after HIFU. At 6, 12 and 24 months after HIFU, 52 %, 63 % and 78 %, respectively, of the patients who had not received neoadjuvant hormonal therapy were potent.
In a prospective study, Li et al. compared the IIEF score, penile colour Doppler ultrasound, penile length and circumference on patients treated for PCa with HIFU or cryoablation (Li et al. 2010). A total of 55 patients in the HIFU group and 47 in the cryoablation group were included. At 36 months, cryoablation patients experienced a lower erectile function recovery rate compared to HIFU patients (Cryoablation = 46.8 %; HIFU = 65.5 %; p = 0.021). No significant decreases in penile length and circumference were found in the two groups (all p-values ≥0.05). Finally, HIFU treatment seems to be standardized with similar outcomes between centers (Rebillard et al. 2003).
2.4.2 Salvage After HIFU Failure
2.4.2.1 HIFU Retreatment
One of the potential interests of HIFU, especially compared to radiotherapy, is the fact that treatment can be repeated. Unlike radiation, there is no dose limitation and no limited number of sessions. In case of incomplete treatment or treatment failure, HIFU does not result in a therapeutic impasse. The re-treatment rate is estimated in the literature to be between 1.2 and 1.47 % (Uchida et al. 2006a, b, c; Crouzet et al. 2010a, b, c; Thuroff et al. 2003; Blana et al. 2006). Morbidity related to repeat HIFU treatment for localized PCa has been studied on 223 patients with a re-treatment rate of 22 %. While urinary infection, bladder outlet obstruction and chronic pelvic pain did not significantly differ after one or more sessions; a significant increase was observed for urinary incontinence and impotence in the group, which required retreatment (Blana et al. 2006).
2.4.2.2 External Radiation Therapy (ERBT)
EBRT is feasible after HIFU. In a retrospective study, Pasticier et al. (2010) included patients treated with salvage radiation after HIFU. A total of 100 patients were included with a median follow-up of 33 months. Mean doses of radiation were 71.9 ± 2.38 Gys. 83 patients underwent radiation treatment only and 17 patients underwent radio-hormonal treatment. The mean time between HIFU and ERBT was 14.9 ± 11.8 months. Mean PSA before salvage ERBT was 2.1 ± 1.8 ng/mL and the nadir PSA after ERBT was 0.28 ± 0.76 ng/mL, with 17.4 ± 10.8 months to reach nadir. The incontinence rate was the same both before and 1 year after salvage ERBT. The progression-free survival rate was 76.6 % at 5 years, and was 93, 70 and 57.5 % for low, intermediate and high-risk groups, respectively. The predicting factors of failure were the PSA nadir after salvage ERBT and the time to reach nadir after ERBT. Recently, similar results were published by Ripert et al. (2011) which reported the disease free survival rate after HIFU was 3 % at 36.5 months (Phoenix criteria) and there was no major EBRT related toxicity at 12 or 24 months.
2.4.2.3 Salvage Surgery
Salvage surgery is feasible after HIFU but with higher morbidity than after primary surgery. Lawrentschuk et al. (2011) reported the results in 15 men with a rising PSA and biopsy-verified PCa after HIFU treatment. Perioperative morbidity was limited to one transfusion in a patient with a rectal injury. Pathological extensive peri-prostatic fibrosis was found in all patients. Postoperative PSA value was undetectable in 14 patients (93.3 %). Six out of 10 patients experienced no postoperative incontinence at 12 months, but with uniformly poor erectile function. Salvage surgery after HIFU is difficult to perform due to fibrotic reaction. In selected patients with a long life expectancy, only experienced surgeons should perform salvage surgery after HIFU.
2.4.3 Salvage HIFU After ERBT or Brachytherapy
External radiation therapy (ERBT), or brachytherapy, is used as a curative treatment of localized PCa. Several studies have shown that tumor destruction is not complete (Kirkham et al. 2008). Given these tumor recurrences, there is no clear consensus on selecting optimal therapeutic management. Most often a hormonal treatment is implemented to delay the onset of metastasis. Recovery techniques exist, including radical prostatectomy, cryotherapy and brachytherapy, but they are technically difficult and induce significant side effects. The efficacy of HIFU was evaluated as salvage treatment following radiotherapy failure to identify pre-operative predictive factors of success.
2.4.3.1 ERBT Failure
The rate of positive biopsy after External Beam Radio Therapy (ERBT) for PCa in the literature is between 25 and 32 % (Borghede et al. 1997; Zelefsky et al. 2001). There appears to be curative intents for salvage HIFU therapy for patients with a locally proven recurrence after external-beam radiation therapy (patients presenting no metastases) and for patients that are usually treated with androgen deprivation (AD). Local control was achieved with negative biopsies in 73 % of cases, with a median PSA nadir of 0.19 ng/mL (Murat et al. 2009). A mean follow-up of 18.1 (3–122) months was reported, the overall actual 5-year specific survival rate being 84 %. The actual 3-year progression-free survival (PSA greater than nadir + 2 ng/mL, positive biopsy or salvage treatment requirement) was 53, 43 and 25 % for low- and intermediate-risk patients, respectively, according to D’Amico’s risk groups. Disease progression was inversely related to pre-HIFU PSA and the use of AD during PCa management. In a recent study, we examined the outcomes of salvage HIFU in 290 consecutive patients (Crouzet et al. 2012). The mean PSA nadir post-HIFU was 1.54 ± 3.38 ng/mL (median 0.14). The estimated cancer-specific and metastasis free survival rates at 5 and 7 years were 80 % (95 % CI 72.7–88.5 %) and 79.6 % (95 % CI 73.5–86.2 %), respectively. In the multivariate analysis three factors were significantly linked to disease progression. The increase of the Progression Free Survival Rate (PFSR) with the pre-HIFU PSA level was statistically significant (p = 0.0002). Previous AD treatment increased the PFSR by a factor of 1.3 (p = 0.01), and a Gleason score over or equal to 8 increased it by a factor of 1.2 (p = 0.01), compared to a Gleason score of less than or equal to 6. While the technique offers promising results, it has to be weighed against the side effects. Since 2002, the Ablatherm® device has included specific acoustic parameters for salvage HIFU. The acoustic dose was adapted to the low blood flow inside the gland fibrosis induced by radiation. Concerning incontinence, 54 % of the patients had no incontinence after salvage HIFU, whereas 25 % had a grade I incontinence (no pads + grade I = 79 %). The risk of urethrorectal fistulas (URF) was only 0.4 % with the introduction of a specific treatment algorithm designed for radiation failure. The impotence rate increased from 36.9 % before salvage HIFU to 58.7 % after treatment (Berge et al. 2010). With the Sonablate®, the biochemical survival rate was 71 % at 9 month (Zaracharakis et al. 2008) and 52 % at 5 years (Uchida et al. 2010). Nevertheless, the risk–benefit ratio of salvage HIFU compares favourably with those of the other available techniques and with less morbidity and similar oncological outcomes. In this context, HIFU appears to be an effective curative treatment option for local recurrence after radiation failure.
2.4.3.2 Brachytherapy Failure
Sylvester et al. (2010) reported a 15-year biochemical relapse-free survival rate and cause-specific survival following Iodine-125 prostate brachytherapy in 215 patients: 15-year biochemical relapse free survival (BRFS) for the entire cohort was 80.4 % and the cancer specific survival rate was 84 %. There was no significant difference between the low and intermediate risk groups. Salvage surgery is a challenging procedure after Brachytherapy (Heidenreich et al. 2010). A study with the Ablatherm® device is currently being conducted in Lyon and includes 26 patients (mean age 67 years) with MRI and biopsy-proven recurrence after brachytherapy (non-published data). Nineteen of them underwent whole gland ablation and 7 underwent focal therapy (hemiablation). The mean follow-up was 19 months. The mean PSA before HIFU was 5.02 ± 4.8 ng/mL, (median PSA 0.35 ng/mL). Nine patients have undetectable PSA with no hormonal deprivation treatment, 8 needed hormonal deprivation treatment for a rising PSA and 9 are recent cases with a very short follow-up. The complication rate was high in the first 9 cases with 3 urinary incontinences (grade 3) and 1 urethrorectal fistula. For these first patients, we used the treatment acoustic parameters defined for radiation failure. Due to the high rates of rectal injury and severe incontinence, new specifically designed treatment parameters for brachytherapy failure were developed with a decrease in the acoustic dose according to the intense prostate fibrosis. Since the introduction of these new parameters, no urethrorectal fistula occurred and no rectal lesion was seen on control MRI, all while maintaining same treatment efficacy.
2.5 Focal Therapy with HIFU
Standard treatment for PCa has long been “whole-gland” therapy or radiation therapy of the entire prostate. It would be interesting, however, to destroy only the cancer foci in order to decrease the treatment morbidity while maintaining its oncologic efficacy. This so-called ‘focal therapy’ can be performed using several techniques: Cryotherapy, HIFU, Brachytherapy and Interstitial Laser Therapy, either with or without photodynamic therapy (PDT). HIFU might be one of the best techniques for focal therapy because it is performed under real-time control using ultrasound or MRI. Immediate control of the necrotic area boundaries is possible using contrast agents, either with ultrasound or MRI. HIFU can also be repeated if necessary. Finally, several salvage standard curative therapies are feasible after focal HIFU.
The first condition for ‘focal therapy’ is to precisely determine the size and position of the different tumor foci within the gland before the ablation. Standard biopsy protocols cannot provide an accurate detection of all tumor foci. Transperineal template biopsies may increase cancer detection. However, the precision of the location within the gland of the cancers detected with this method remains difficult to assess. Additionally, template biopsy requires general anaesthesia or heavy sedation and is associated with increased morbidity, with at least 10 % of urinary retention due to oedema and bleeding (Rouviere et al. 2012a). Ideally, the simplest way to select patients for focal therapy would be to use imaging. Imaging could also be used to assess whether the target area has been correctly destroyed and to detect for local recurrences. Nonetheless, its role in focal therapy remains under debate.
2.5.1 The Current Role of Imaging in PCa Focal Therapy
In theory, imaging could be used in three different fields: To detect and localize PCa within the gland, to assess tissue destruction after HIFU ablation and to detect post-HIFU cancer recurrence.
2.5.1.1 Patient Selection and Treatment Planning: The Need for Better PCa Mapping
For many years, prostate imaging has yielded suboptimal results in PCa detection and localization, and the results of US-based techniques have been particularly disappointing (Rouvière et al. 2007).
Nevertheless, excellent results have recently been published with MRI, especially since dynamic contrast-enhanced (DCE) and diffusion-weighted (Dw) sequences have been used in addition to the classical T2-weighted (T2w) imaging. There is now a large and concordant body of literature showing that this so-called prostate ‘multiparametric MRI’ (mp-MRI) allows for good detection of high-grade PCas (Gleason score ≥7), with an excellent negative predictive value in candidates undergoing radical prostatectomy (Girouin et al. 2007; Villers et al. 2006; Turkbey et al. 2010; Bratan et al. 2013), but also in the more challenging population of candidates undergoing prostate biopsy (Cheikh et al. 2009; Habchi et al. 2014) (Fig. 2.4).
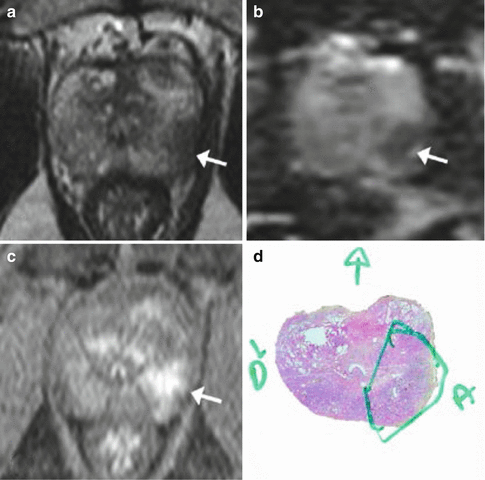

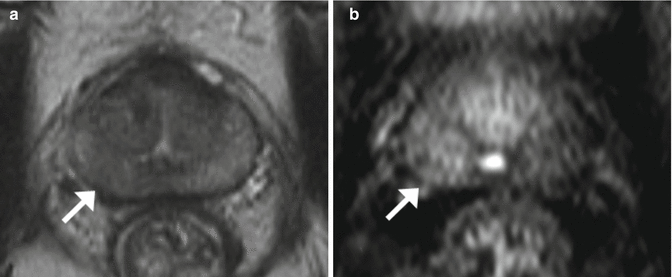
Fig. 2.4
MRI: Multiparametric MRI obtained in a 59-year old patient with a PSA level of 6 ng/mL presenting a suspicious lesion in the peripheral zone of the left mid-gland, with marked hyposignal on the T2-weighted imaging (a, arrow). Apparent Diffusion Coefficient map (b, arrow) and early and marked enhancement on Dynamic Contrast-Enhanced imaging (c, arrow). Radical prostatectomy confirmed the presence of a Gleason 8 (4 + 4) cancer (d)
The detection of anterior tumors, which are usually missed by random biopsies, is also excellent (Lemaitre et al. 2009). A recent study reported the results of precise radiopathological correlations in 175 patients treated by radical prostatectomy following preoperative mp-MRI (CLARA-P database). MR detection rates for tumors of <0.5 cc, 0.5–2 cc and >2 cc were respectively 21–29 %, 43–54 % and 67–75 % for Gleason ≤6 cancers, 63 %, 82–88 % and 97 % for Gleason 7 cancers, and 80 %, 93 % and 100 % for Gleason ≥8 cancers (Bratan et al. 2013). These results suggest that mp-MRI is an excellent screening tool, with a good negative predictive value for Gleason ≥7 tumors. However, the detection of Gleason ≤6 tumors remains limited.
In addition, mp-MRI specificity needs to be improved since up to 40 % of MR abnormalities are benign (Bratan et al. 2013; Cheikh et al. 2009; Habchi et al. 2014; Lemaitre et al. 2009; Rouviere et al. 2012a, b). To improve the characterization (benign/malignant) of focal lesions detected by mp-MRI, it has been suggested that a five-level Likert score (1, definitely benign; 2, likely benign; 3, indeterminate; 4, likely malignant; 5, definitely malignant) be used. Although subjective and entirely based on the radiologist’s experience, this score has been proved to significantly stratify the likelihood of malignancy of MR lesions. In the CLARA-P database, the percentage of malignant lesions was 7–26 %, 27–41 %, 61–72 % and 97–98 % in MR lesions with a Likert score of 2/5, 3/5, 4/5 and 5/5, respectively (Bratan et al. 2013). Semi-objective scores based on more precise features have been described recently (Rouviere et al. 2012a, b; Barentsz et al. 2012; Puech et al. 2013). Particularly, the European Society of Urogenital Radiology (ESUR) endorsed the Prostate Imaging Reporting and Data System (PIRADS) that assigns a score ranging from 3 to 15 based on T2w, Dw and DCE imaging (Barentsz et al. 2012). However, it remains unclear whether these semi-objective scores perform better than the subjective Likert score. Two recent studies have even shown that, paradoxically, the Likert score yielded better inter-observer agreement than the PIRADS score (Rosenkrantz et al. 2013; Vache et al. 2014). Thus, new scores will probably be defined in the future to improve the characterization of MR abnormalities seen at mp-MRI. Some authors also used computer-aided diagnostic systems with interesting results on preliminary studies (Hambrock et al. 2013; Niaf et al. 2014).
Focal ablation also requires a good evaluation of the tumor volume. Little has been published on mp-MRI accuracy in assessing tumor volume. Several studies have, however, pointed out that mp-MRI had a tendency to underestimate tumor volume (Cornud et al. 2014; Le Nobin et al. 2014). The optimal safety margin to use around malignant lesions seen on mp-MRI remains to be determined.
After radiation therapy, mp-MRI showed excellent results in detecting and localizing local recurrences (Cornud et al. 2014; Le Nobin et al. 2014; Rouvière et al. 2004; Haider et al. 2008; Donati et al. 2013; Roy et al. 2013). Tumor detection appears easier than in untreated prostates because of the favourable contrast between recurrent cancer and post-radiation fibrosis on DCE and Dw imaging (Fig. 2.5).