Prostate Implants: Technique, Dosimetry, and Treatment Planning
24.1. INTRODUCTION
Treatment options for carcinoma of the prostate include radical prostatectomy (e.g., nerve-sparing surgical procedure), external photon beam irradiation, and brachytherapy implantation. The selection of a particular procedure or a combination of procedures depends on established prognostic factors such as stage and grade. In general, surgery is indicated if the tumor is confined to the prostate gland with no extension through the capsule or into the seminal vesicles. Implants are used for early-stage cancers, either alone or in conjunction with external beam radiation therapy. However, patients with extensive tumors (TNM stage T3 and T4) are not good candidates for implantation.
Except as general information, clinical aspects of prostate gland cancer and its treatment are beyond the scope of this book. The reader is referred to a wealth of information on this subject in the medical literature. In this chapter, we will discuss the physical and technologic aspects of prostate gland implants using radioactive seeds and high-dose-rate (HDR) brachytherapy.
24.2. SEED IMPLANTS
Two types of seed implants have been used for prostate gland: a temporary implant and a permanent implant. The temporary implants involve radioisotopes of relatively long half-life and sufficient dose rate to deliver the prescribed target dose in 3 to 4 days. The sources are removed at the end of that period. In the permanent implant, the radioisotope emits photons of low enough energy that the radiation from the patient poses no significant hazard to persons in the surrounding environment. The sources are left in the patient forever, and the prescribed dose is delivered during complete decay of the sources. Of these two types of seed implants, the permanent implants are predominant and will be discussed in greater detail.
A. PERMANENT IMPLANTS
Permanent implants with iodine-125 or palladium-103 are used in the treatment of early-stage prostate cancer as the sole modality or in combination with external beam radiation therapy. The target volume for implantation in either case is the prostate gland itself, with minimal margins allowed to account for uncertainty of prostate localization.
Whitmore and Hilaris pioneered prostatic implantation with 125I seeds in the early 1970s at Memorial-Sloan Kettering Cancer Center. They used the retropubic approach, which entailed a major surgical procedure. The treatment results were disappointing and so, by the mid-1980s, the retropubic technique was abandoned.
The modern technique of implantation, which began in the 1980s, consists of a transperineal approach in which 125I or 103Pd seeds are inserted into the prostate gland with the guidance of transrectal ultrasonography and perineal template. The procedure is nonsurgical and performed on an outpatient basis. The implant is done in an approved operating room with the patient requiring a spinal anesthetic.
A.1. Volume Study
Localization of the prostate by a series of transverse ultrasound images constitutes a volume study. The patient is placed in the dorsal lithotomy position and the transrectal ultrasound probe (5- to 6-MHz transducer) is securely anchored. The probe is moved precisely to obtain transverse images of the prostate gland from base to apex at 5-mm intervals. A grid is superimposed on each image to represent template coordinates. The prostate gland is visualized on each of the transverse images and the implantation target is drawn to encompass the prostate. The sagittal image is also obtained to measure the length of the gland from the base to the apex. This provides a double check of the number of transverse images and the number of seeds required for the central needle. Prior to the volume study, evaluation is made from computed tomography (CT) scans of the prostate gland size and the pubic arch in relation to the prostate. If the pubic arch is too narrow, it will prevent the needles from reaching the target. In the case of a large gland and significant pubic arch interference, the patient may need hormonal therapy for a few months to shrink the gland to allow for an adequate implant. Some radiation oncologists prefer hormonal therapy in most cases to reduce the gland size to minimize technical problems of implantation.
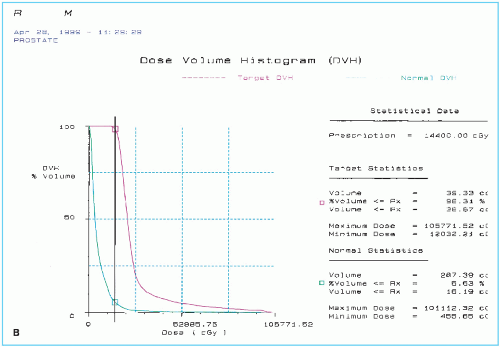
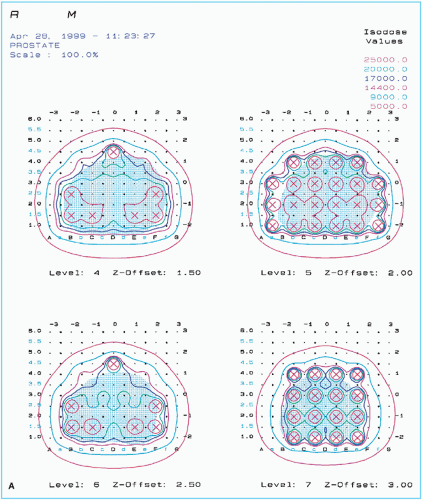 Figure 24.1. A sample of pretreatment plan with 125I seeds showing (A) seeds and isodose curves in four ultrasound cross sections of prostate gland and (continued) |
A.2. Treatment Planning
A treatment-planning system specifically designed for prostate gland implants allows the target outlines from the volume study to be digitized into the computer. The implant is planned with an interseed spacing of 1 cm (center to center) and a needle spacing of 1 cm. The computer software allows the placement of seeds in the template grid in each of the ultrasound images. Individual seeds can be added or deleted iteratively to optimize isodose coverage of the target volume. Seed strength can be adjusted to deliver a prescribed minimum peripheral dose (MPD), which is the isodose surface just covering the prostate target volume. Before the availability of computer dosimetry, seed strength and the required number of seeds were determined by the method of dimension averaging used in conjunction with a precalculated nomogram (1). The modern computer programs allow the use of any seed strength as well as fine adjustment of this parameter to obtain the desired MPD. Typical seed strengths required are on the order of 0.3 mCi for 125I (MPD = 144 Gy) and 1.7 mCi for 103Pd (MPD = 125 Gy).
Based on the approved computer plan, a worksheet is prepared specifying the number of needles, seeds in each needle, and template coordinates. Figure 24.1 shows an example of preimplant treatment plan for 125I along with the dose volume histogram and statistics. In evaluating the preimplant plan, distribution of seeds and isodose curves are reviewed in each ultrasound cross section to assure adequate coverage of the target volume and sparing of normal tissue. Dose volume histograms for the target and the critical structures are useful to provide overall statistical evaluation of the treatment plan.
Critical structures or organs at risk in a prostate implant are the urethra, rectum, and bladder. Seeds should not be placed directly in or in close proximity of these structures because doses are extremely high at or very close to a brachytherapy source. These structures must be outlined in the plan so that they can be viewed in the isodose plans and DVHs. Careful planning is important to avoid high doses to these structures.
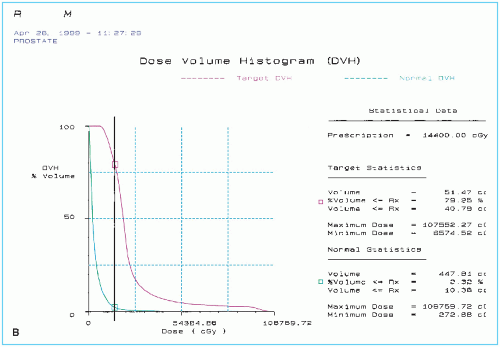
Postimplant dosimetry may also be performed using CT scans to assess stability of the implant after swelling of the prostate gland has gone down. A major problem with permanent seed implants is the usual disagreement between the preimplant and postimplant dose distributions. Hot and cold spots can develop as a result of source movement with time (Fig. 24.2), leaving one to wonder if the prescribed dose was delivered to the target accurately with a pattern of dose distribution as originally planned and that the critical structures were spared as intended.
Another equally serious problem is that of source anisotropy. Because of the low γ-ray energy emitted and the design of the source in which radiation is severely attenuated along the length of the seed, cold spots of greater than 50% (reduction in dose) exist at the ends (see Figs. 15.4 and 15.6). This anisotropy in dose distribution, however, is more of a problem if the sources are aligned permanently end to end with each other along straight lines. A certain degree of randomness that naturally develops after implantation reduces the overall anisotropy effect in a prostate gland implant.
A.3. Implant Procedure
The implant procedure is carried out as an outpatient treatment in an operating room with the patient in the dorsal lithotomy position under spinal anesthesia. Figure 24.3 shows the implantation apparatus consisting of a transrectal ultrasound probe and a template to guide specifically designed sterile 18-gauge, 21-cm-long needles. The needles are preloaded with the planned number of seeds and spacers and placed in a needle holder at appropriate template coordinates. The needle-loading procedure is performed behind an L-shaped leaded glass barrier. Each needle is equipped with a plunger and the tip is sealed with bone wax to keep the seeds in place until implantation.
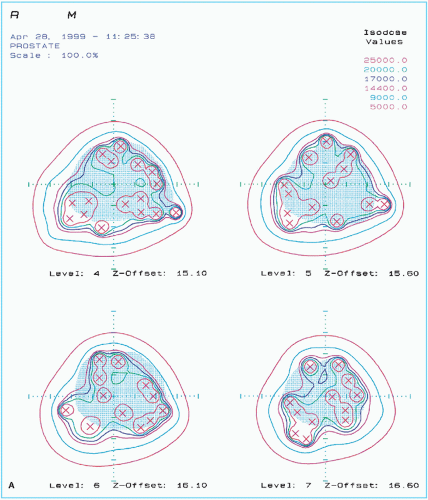 Figure 24.2. A sample of posttreatment plan of the same patient as in Figure 24.1, showing (A) seeds and isodose curves in the four ultrasound cross sections of the prostate gland and (continued) |
The needles are inserted one at a time into the prostate using the ultrasound and template guidance. In each case, using sagittal images and distance measurements from the hub of the needle to the template, it is ascertained that the needle tip is at the correct plane and depth. After verifying the needle position, the needle is slowly withdrawn while the plunger is held stationary. This action results in the injection of the seeds and the spacers into the tissues along the track of the withdrawing needle. Each ultrasound image is carefully reviewed to assess the position of the seeds. Final verification of the implant is made with anteroposterior fluoroscopy. Extra seeds are available for implantation if cold spots are identified. Cystoscopy is performed at the conclusion of the procedure to retrieve any stray seeds in the bladder or the urethra.
A.4. Radiation Protection
The American Association of Physicists in Medicine (AAPM) code of practice for brachytherapy (4) is a comprehensive document on the physics and quality assurance of brachytherapy




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree