Psychiatric Disorders
Marc Laruelle
Anissa Abi-Dargham
Psychiatric conditions were once qualified as “functional” illnesses, that is, disorders in which no major abnormalities of brain integrity such as tumors, inflammation, or infection could be detected by neuropathological examination. Indeed, following a century of postmortem studies, only subtle abnormalities have been found at autopsy in brains of patients who suffered from major psychiatric illnesses, and few of these have been consistently replicated. These observations led to the impression that these illnesses were due to functional rather than structural brain abnormalities. Computed tomography (CT) and magnetic resonance imaging (MRI) studies have consistently observed abnormalities in brain regional volumes in many of these conditions, but these abnormalities remain for the most part within the limits of normal variability. None of them is pathognomonic or diagnostic. Therefore, the advent of molecular imaging with positron emission tomography (PET) and single-photon emission computed tomography (SPECT) held enormous promises for the field of psychiatry because, for the first time, living brain functions were directly accessible to clinical investigation.
Using PET and SPECT, a large body of studies has documented that psychiatric disorders are associated with regional alterations in flow and metabolism under both resting and activation conditions. In this regard, PET and SPECT have played a major role in unraveling alterations in brain function associated with these conditions. Today these techniques have largely been replaced by functional MRI (fMRI), which offers clear advantages in terms of spatial and temporal resolution, not to mention the lack of radiation exposure. Therefore, it is foreseeable that the role of PET and SPECT studies of flow and, to some extent, metabolism in psychiatry research may be greatly reduced in the future.
On the other hand, the ability of nuclear medicine techniques to image specific biomolecules is unmatched by any other method currently available to clinical investigators. Studies of receptors, transporters, enzymes, and other processes such as transmitter release clearly constitute the uniqueness of PET for current and future psychiatric research. These techniques have already yielded a number of fundamental observations. So far none of these findings has led to clinical applications useful in the diagnosis or treatment of these disorders in individual patients. However, it is anticipated that such applications may surface from this line of research in the near future.
The aim of this chapter is to describe the major findings stemming from this line of research and their implications for our understanding of the pathophysiology and treatment of major psychiatric illnesses. For the reasons discussed above, the chapter will focus on imaging studies of specific biomolecules as opposed to the study of flow and metabolism. Nonetheless, important flow and metabolism studies will be discussed, particularly when molecular imaging studies provide clues to the pathophysiology underlying their observations. The chapter will include both PET and SPECT studies, as it would not be feasible to provide a comprehensive review of this field without describing the important contributions of SPECT.
Technical considerations critical to the discussion of the findings will be included when appropriate, but, for an overview of the technical background of these studies, the reader is referred to Chapters 1–3, and 5 in this book. In line with the clinical orientation of this chapter, the studies will be reviewed by disorder (schizophrenia, mood, anxiety, personality, conduct, and substance abuse disorders) rather than by transmitter system.
The main neurotransmitter systems that have been studied with PET or SPECT in relation to psychiatric disorders and their treatment include dopamine (DA), serotonin (5-HT), γ-aminobutyric acid (GABA), and opiate systems. Radiotracers most frequently used include those for the DA D2/3 receptor: carbon-11 [11C]-N-methylspiperone, [11C]-raclopride, iodine-123 [123I]-IBZM (benzamide derivative (S)-3-[123l]-iodo-N-[(1-ethyl-2-pyrrolidinyl)])-methyl-2-hydroxy-6-methoxybenzamide), [123I]-epidipride, fluorine-18 [18F]-fallypride, [11C]-FLB457, [11C]-PHNO (11)C](+)-PHNO ([(11)C](+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b] [1,4]oxazin-9-ol; D1 receptors: [11C]-SCH23390, [11C]-NNC112; DA transporters: [11C]-cocaine, [11C]-methylphenidate, [123I]-β-CIT (123I-labeled 2-β-carbomethoxy-3-β-(4-iodophenyl)-tropane),
[11C]/[18F]-CFT (2-β-carbomethoxy-3-β-(4-fluorophenyl) tropane), technetium-99m [99mTc]-TRODAT-1; dopa decarboxylase: [18F]/[11C]-DOPA; monoamine oxydase: [11C]-deprenyl and [11C]-clorgyline; 5-HT2 receptors: [18F]-altanserin, [18F]-setoperone, [11C]-MDL100907; 5-HT1A receptors: [11C]-WAY100635; 5-HT transporters: [123I]-β-CIT, [11C]-McN5652, [11C]-DASB; benzodiazepine receptors: [123I]-iomazenil, [11C]-flumazenil; and mu opiate receptors: [11C]-carfentanil, [18F]-cyclofoxy.
[11C]/[18F]-CFT (2-β-carbomethoxy-3-β-(4-fluorophenyl) tropane), technetium-99m [99mTc]-TRODAT-1; dopa decarboxylase: [18F]/[11C]-DOPA; monoamine oxydase: [11C]-deprenyl and [11C]-clorgyline; 5-HT2 receptors: [18F]-altanserin, [18F]-setoperone, [11C]-MDL100907; 5-HT1A receptors: [11C]-WAY100635; 5-HT transporters: [123I]-β-CIT, [11C]-McN5652, [11C]-DASB; benzodiazepine receptors: [123I]-iomazenil, [11C]-flumazenil; and mu opiate receptors: [11C]-carfentanil, [18F]-cyclofoxy.
Schizophrenia
Dopamine Transmission
The classical DA hypothesis, formulated over 40 years ago, proposed that schizophrenia is associated with hyperactivity of dopaminergic neurotransmission (1,2). This hypothesis was essentially based on the observation that all effective antipsychotic drugs provided at least some degree of D2 receptor blockade (3,4), an observation that is still true today. As D2 receptor blockade is most effective against positive symptoms (delusions and hallucinations), the DA hyperactivity model appeared to be most relevant to the pathophysiology of these symptoms. This idea was further supported by the fact that sustained exposure to DA agonists such as amphetamine can induce a psychotic state characterized by some features of schizophrenic positive symptomatology (emergence of paranoid delusions and hallucinations in the context of a clear sensorium) (5,6). These pharmacological effects suggest, but do not establish, a dysregulation of DA systems in schizophrenia.
On the other hand, negative and cognitive symptoms are generally resistant to treatment by antipsychotic drugs. Functional brain imaging studies have suggested that these symptoms are associated with prefrontal cortex (PFC) dysfunction (7). Studies in nonhuman primates have demonstrated that deficits in DA transmission in the PFC produce cognitive impairments reminiscent of those observed in schizophrenic patients (8), suggesting that a deficit in DA transmission in the PFC may be implicated in the cognitive impairments associated with schizophrenia (9,10).
Thus, a contemporary view of the role of DA in schizophrenia is that subcortical mesolimbic DA projections may be hyperactive (resulting in positive symptoms) and that the mesocortical DA projections to the PFC may be hypoactive (resulting in negative symptoms and cognitive impairment). Furthermore, these two abnormalities may be related, as the cortical DA system generally exerts an inhibitory action on subcortical DA systems (11,12). The advent in the early 1980s of techniques based on PET and SPECT to measure indices of DA activity in the living human brain opened the possibility of direct investigation of these hypotheses.
Subcortical Dopamine Transmission
Studies of striatal DA transmission in schizophrenia examined both postsynaptic (D2 receptors and D1 receptors) and presynaptic ([DOPA] decarboxylase activity, stimulant-induced DA release, baseline DA release, and dopamine transporter [DAT]) functions.
Striatal Dopamine Receptors
Striatal D2 receptor density in schizophrenia has been extensively studied with PET and SPECT imaging (unless specified otherwise, the term D2 receptor is used in this chapter to designate both D2 and D3 receptors). A meta-analysis (13) of these studies identified 17 studies comparing D2 receptor parameters in patients with schizophrenia (included a total of 245 patients, 112 neuroleptic naive, and 133 neuroleptic free), and controls (n = 231), matched for age and sex (14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30). Radiotracers included butyrophenones ([11C]-N-methyl-spiperone, [11C]-NMSP, n = 4 studies, and bromine-76 [76Br]-bromospiperone, n = 3 studies), benzamides ([11C]-raclopride, n = 3 studies, and [123I]-IBZM, n = 5 studies) or the ergot derivative [76Br]-lisuride, n = 2 studies). Only 2 of 17 studies detected a significant elevation of D2 receptor density parameters. However, meta-analysis revealed a small (12%) but significant elevation of striatal D2 receptors in patients with schizophrenia. No clinical correlates of increased D2 receptor binding parameters have been reliably identified. Studies performed with butyrophenones (n = 7) show an effect size of 0.96 ± 1.05, significantly larger than the effect size observed with other ligands (benzamides and lisuride, n = 10; 0.20 ± 0.26; P = .04). This difference has been attributed to differences in vulnerability of the binding of these tracers, to competition by endogenous DA, and to elevation of endogenous DA in schizophrenia (31,32). Interestingly, a recent study in unaffected monozygotic twins of patients with schizophrenia suggested that a modest elevation of D2 receptors in the caudate might be associated with genetic vulnerability to schizophrenia (33).
Regarding striatal D1 receptors, several imaging studies have confirmed the results of postmortem studies of unaltered levels of these receptors in the striatum of patients with schizophrenia (20,34,35).
Several lines of evidence suggest that D3 receptors might play an important role in the pathophysiology and treatment of schizophrenia (36). An increase in the concentration of D3 receptors has been reported in postmortem brains of patients with schizophrenia (37). In addition to their role in schizophrenia, D3 receptors are also believed to play an important role in drug addiction (38). Until recently, imaging D3 receptor was not feasible: PET radiotracers commonly used to study D2 and D3 receptors exhibit similar affinities for both receptors and the concentration of D3 receptors in the human striatum is lower than that of D2 receptors. The recent discovery that [11C]-PHNO is a D3 preferring imaging agent might open a window to the in vivo study of this important target (39).
Striatal DOPA Decarboxylase Activity
Several studies have reported rates of activity of DOPA decarboxylase in patients with schizophrenia, using [18F]-DOPA (40,41,42,43,44,45) or [11C]-DOPA (46). The majority of these studies reported increased accumulation of DOPA in the striatum of patients with schizophrenia, and the combined analysis yielded a significant effect size (P = .01) (13). Together, these studies provide the strongest evidence for the existence of a dysregulation of DA function in the striatum of untreated patients with schizophrenia. In addition, several of these studies reported that high DOPA accumulation was more pronounced in psychotic paranoid patients, while low accumulation was observed in patients with negative or depressive symptoms and catatonia. Although the relationship between DOPA decarboxylase and the rate of DA synthesis is unclear (DOPA decarboxylase is not the rate-limiting step of DA synthesis), these observations are compatible with higher DA synthesis activity of DA neurons in schizophrenia, at least in subjects experiencing psychotic symptoms.
Striatal Amphetamine-induced Dopamine Release
D2 receptor imaging, combined with pharmacological manipulation of DA release, enables more direct evaluation of DA presynaptic activity. Numerous groups have demonstrated that an acute
increase in synaptic DA concentration is associated with decreased in vivo binding of benzamide radioligands, such as [11C]-raclopride, [18F]-fallypride, or [123I]-IBZM. These interactions have been demonstrated in rodents, nonhuman primates, and humans, using a variety of methods to increase synaptic DA (for review of this abundant literature, see Laruelle [47]). It has also been consistently observed that the in vivo binding of spiperone and other butyrophenones is not as affected by acute fluctuations in endogenous DA levels, as is the binding of benzamides (47).
increase in synaptic DA concentration is associated with decreased in vivo binding of benzamide radioligands, such as [11C]-raclopride, [18F]-fallypride, or [123I]-IBZM. These interactions have been demonstrated in rodents, nonhuman primates, and humans, using a variety of methods to increase synaptic DA (for review of this abundant literature, see Laruelle [47]). It has also been consistently observed that the in vivo binding of spiperone and other butyrophenones is not as affected by acute fluctuations in endogenous DA levels, as is the binding of benzamides (47).
The decrease in [11C]-raclopride and [123I]-IBZM in vivo binding following acute amphetamine challenge has been well validated as a measure of the change in D2 receptor stimulation by DA due to amphetamine-induced DA release. Manipulations that are known to inhibit amphetamine-induced DA release, such as pretreatment with the DA synthesis inhibitor α-methyl-para-tyrosine (αMPT) or with the DAT blocker GR12909, also inhibit the amphetamine-induced decrease in [123I]-IBZM or [11C]-raclopride binding (48,49). The effect of methamphetamine on [11C]-raclopride in vivo binding is also significantly blunted in patients with Parkinson disease (50). Combined microdialysis and imaging experiments in primates demonstrated that the magnitude of the decrease in ligand binding was correlated with the magnitude of the increase in extracellular DA induced by the challenge (26,49), suggesting that this noninvasive technique provides an appropriate measure of the changes in synaptic DA levels.
Three of three studies demonstrated that the amphetamine-induced decrease in [11C]-raclopride or [123I]-IBZM binding was elevated in untreated patients with schizophrenia compared to well-matched controls (24,26,27). A significant relationship was observed between the magnitude of DA release and the transient induction or deterioration of positive symptoms. The increased amphetamine-induced DA release was observed in both first episode/drug-naive patients and patients previously treated with antipsychotic drugs (51). Patients who were experiencing an episode of illness exacerbation (or a first episode of illness) at the time of the scan showed elevated amphetamine-induced DA release, while patients in remission showed DA release values not different from controls (51), suggesting that the dysregulation of the DA system revealed by this challenge might represent a state rather than a trait factor. This exaggerated response of the DA system to amphetamine exposure did not appear to be a nonspecific effect of stress, as elevated anxiety before the experiment was not associated with a larger amphetamine effect. Furthermore, nonpsychotic subjects with unipolar depression, who reported levels of anxiety similar to the schizophrenic patients at the time of the scan, showed normal amphetamine-induced displacement of [123I]-IBZM (52).
These findings were generally interpreted as reflecting a larger DA release following amphetamine in the schizophrenic group. Another interpretation of these observations would be that schizophrenia is associated with increased affinity of D2 receptors for DA. Over the past few years, several D2 receptor radiolabeled agonists such as [11C]-NPA ([11C]N-propyl-norapomorphine) and [11C]-PHNO have been successfully developed, and studies using these agents in patients with schizophrenia are needed to solve this issue (39,53,54,55,56,57).
Striatal Baseline Dopamine Activity
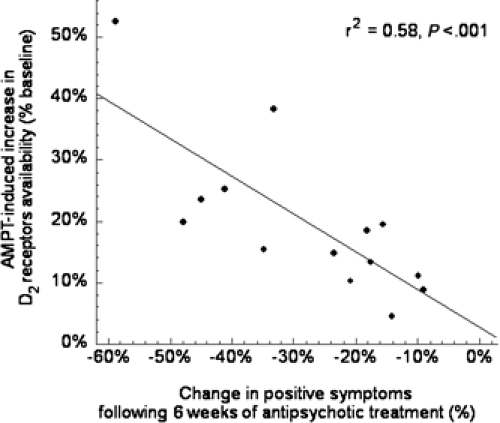
A limitation of the amphetamine challenge of imaging studies is that they measure changes in synaptic DA transmission following a nonphysiological challenge (i.e., amphetamine) and do not provide any information about synaptic DA levels at baseline (i.e., in the unchallenged state). Several laboratories have reported that, in rodents, acute depletion of synaptic DA is associated with an acute increase in the in vivo binding of [11C]-raclopride or [123I]-IBZM to D2 receptors (for review, see Laruelle [47]). The increased binding was observed in vivo but not in vitro, indicating that it was not due to receptor up-regulation (58), but to removal of endogenous DA and unmasking of D2 receptors previously occupied by DA. The acute DA depletion technique was developed in humans using αMPT, to assess the degree of occupancy of D2 receptors by DA (58,59,60,61). Using this technique, higher occupancy of D2 receptors by DA was reported in patients with schizophrenia experiencing an episode of illness exacerbation, compared to healthy controls (28). Again, assuming normal affinity of D2 receptors for DA, the data are consistent with higher DA synaptic levels in patients with schizophrenia. Increased D2 receptor stimulation by DA at intake, as measured with the αMPT paradigm, was predictive of rapid clinical response to antipsychotic drugs (28). This finding illustrates the potential of molecular imaging to predict treatment response (Fig. 10.1).
Striatal Dopamine Transporters
The data reviewed above are consistent with higher DA output in the striatum of patients with schizophrenia, which could be explained by increased density of DA terminals. Since striatal DAT are exclusively localized on DA terminals, this question was investigated by measuring binding of [123I]-β-CIT (62) or [18F]-CFT (63) in patients with schizophrenia. Both studies reported no differences in DAT binding between patients and controls. In addition, Laruelle et al. (62) reported no association between amphetamine-induced DA release and DAT density. Thus, the increased presynaptic output
suggested by the studies reviewed above does not appear to be due to higher terminal density, an observation consistent with postmortem studies that failed to identify alteration in striatal DAT binding in schizophrenia (for references, see Laruelle et al. [62]).
suggested by the studies reviewed above does not appear to be due to higher terminal density, an observation consistent with postmortem studies that failed to identify alteration in striatal DAT binding in schizophrenia (for references, see Laruelle et al. [62]).
Taken together, studies of striatal DA transmission in schizophrenia have provided support for the time-honored DA hypothesis of schizophrenia. As animal data suggest, the antipsychotic effect of D2 receptor antagonism is mediated by blockade of D2 receptors in the mesolimbic as opposed to the nigrostriatal DA system (64,65,66), and future studies will focus on studying striatal subsystems. Recent progress in PET instrumentation has provided the resolution necessary to differentiate the signal from ventral (i.e., limbic) and dorsal (i.e., motor) regions of the anterior striatum (67,68,69).
Extrastriatal D2 Receptors
The development of radiotracers suitable for imaging extrastriatal D2 receptors such as [11C]-FLB497 (70) and [18F]-fallypride (71) enabled the study of D2 receptor transmission in extrastriatal areas. Lower D2 receptor density has been described in untreated schizophrenia in the thalamus in three studies (72,73,74), as well as in the midbrain (75), in temporal cortex (76), and cingulated cortex (77). Additional studies are needed to replicate and extend these findings and explore their pathophysiological basis and significance.
Prefrontal Dopamine Transmission
The majority of DA receptors in the PFC are of the D1 subtype (78,79). Cortical D1 receptors have been studied in schizophrenia using [11C]-SCH23390 (80) and [11C]-NNC112 (81,82). In humans, [11C]-NNC112 provides higher specific to nonspecific ratios compared to [11C]-SCH23390 (82,83), a property that is important for quantification of cortical D1 receptors. It should be noted that both ligands display only moderate in vivo selectivity for D1 relative to 5-HT2A receptors, and that 20% to 30% of cortical binding of both radiotracers correspond to binding to 5-HT2A receptors (84,85).
PET studies with [11C]-SCH23390 reported decreased (20) or unchanged (86) prefrontal D1 receptor availability in untreated patients with schizophrenia. In contrast, a study using [11C]-NNC112 reported increased D1 receptor availability in the dorsolateral PFC (DLPFC) of patients with schizophrenia (35). Interestingly, increased [11C]-NNC112 binding was associated with poor performance on the n-back test of working memory (35). The reason for the discrepancy in the results obtained with [11C]-SCH23390 and [11C]-NNC112 remains to be elucidated, but it is interesting to note that the binding of both radiotracers is differentially affected by endogenous DA competition and receptor trafficking (47). For example, chronic DA depletion in rodents is associated with decreased and increased in vivo binding of [11C]-SCH23390 and [11C]-NNC112, respectively (87). Thus, the contradictory observations of decreased [11C]-SCH23390 binding (20) and increased [11C]-NNC112 binding (35) observed in the PFC in patients with schizophrenia might in fact mean that both represent consequences of a sustained deficit in prefrontal DA function. Much work remains to be done to validate this hypothesis. This point illustrates that the in vivo binding of radiotracers is affected by several factors that are not present in the typical in vitro situation, such as the impact of receptor trafficking on ligand affinity (47). This situation represents both a challenge, because the interpretation of the results is less straightforward, and an opportunity, because more information can be gained on the functions of the living neurons.
Serotonin Transmission
Abnormalities of 5-HT transporters (SERT), 5-HT2A receptors, and, more consistently, 5-HT1A receptors have been described in postmortem studies in schizophrenia (see references in Abi-Dargham and Krystal [88]). However, these postmortem results were generally not confirmed by molecular imaging studies.
The concentration of SERT in the midbrain measured by [123I]-β-CIT is unaltered in patients with schizophrenia (62). This observation was confirmed and extended to thalamus and striatum using the more selective radiotracer [11C]-DASB (89). Yet, SERT imaging agents currently available are not valid to study SERT in areas of relatively low SERT density, such as the PFC, where SERT has been reported to be reduced in three of four postmortem studies in schizophrenia (88).
Decreased 5-HT2A receptors have been reported in the PFC in four of eight postmortem studies (see references in Abi-Dargham and Krystal [88]). Three PET studies in drug-naive or drug-free patients with schizophrenia reported normal cortical 5-HT2A receptor binding (90,91,92), while one study reported a significant decrease in PFC 5-HT2A binding in a small group (n = 6) of drug-naive schizophrenic patients (93).
The most consistent abnormality of 5-HT parameters reported in postmortem studies in schizophrenia is an increase in the density of 5-HT1A receptors in the PFC, reported in seven of eight studies (88). Several groups evaluated the binding of this receptor in vivo with PET and [11C]-WAY100907 in schizophrenia and reported inconsistent results. One study reported increased 5-HT1A binding in the temporal lobe of patients with schizophrenia (94), another study reports decreased binding in the amygdala (95), and a third study failed to detect any changes in regional 5-HT1A availability (96). These inconsistencies indicate that the alterations of 5-HT1A receptors demonstrated in postmortem studies in schizophrenia could not be reliably detected with PET and might not be related to the pathophysiology of the illness.
Gamma-aminobutyric Acid Secretion Transmission
A robust body of findings suggests a deficiency of GABAergic function in the PFC in schizophrenia (for reviews, see Lewis [97] and Benes [98]). In vivo evaluation of GABAergic systems in schizophrenia has so far been limited to evaluation of benzodiazepine receptor densities with SPECT and [123I]-iomazenil, and three of three studies comparing patients with schizophrenia and controls reported no significant regional differences (99,100,101). Although significant correlations between symptom clusters and regional benzodiazepine densities have been observed in some studies, these relationships have not been replicated. Thus, taken together, these studies are consistent with an absence of marked abnormalities of benzodiazepine receptor concentration in the cortex and patients with schizophrenia. Alterations of GABAergic systems in schizophrenia might not involve benzodiazepine receptors (102), or be restricted to certain cortical layers or classes of GABAergic cells that are beyond the resolution of current radionuclide-based imaging techniques.
Antipsychotic Drug Occupancy Studies
Perhaps the most widespread use of neuroreceptor imaging in schizophrenia over the past two decades has been the assessment of
receptor occupancy achieved by typical and atypical antipsychotic drugs, a topic that has been reviewed elsewhere (103,104). The main focus has been on D2 receptor occupancy, but 5-HT2A and D1 receptors have also been studied. Studies have repeatedly confirmed the existence of a threshold of occupancy of striatal D2 receptors (about 80%) above which extrapyramidal side effects (EPS) are likely to occur (105). In general, studies have failed to observe a relationship between the degree of D2 receptor occupancy and clinical response (106,107). However, most studies were performed at doses achieving more than 50% occupancy. Two studies performed with low doses of relatively selective D2 receptor antagonists (haloperidol and raclopride) suggested that 50% to 60% occupancy was required to observe a rapid clinical response (108,109). Clozapine, at clinically therapeutic doses, has been found to achieve only 40% to 60% D2 receptor occupancy (105,107,110), which, in conjunction with its anticholinergic properties, may account for its low risk for EPS. Occupancy of 5-HT2A receptors by “5-HT2A/D2 balanced antagonists” such as risperidone does not confer protection against EPS, since the threshold of D2 receptor occupancy associated with EPS is not markedly different between these drugs and drugs devoid of 5-HT2A antagonism (111,112,113,114). Studies with quetiapine suggest that, at least with this agent, transient high occupancy of D2 receptors might be sufficient to elicit a clinical response (115,116).
receptor occupancy achieved by typical and atypical antipsychotic drugs, a topic that has been reviewed elsewhere (103,104). The main focus has been on D2 receptor occupancy, but 5-HT2A and D1 receptors have also been studied. Studies have repeatedly confirmed the existence of a threshold of occupancy of striatal D2 receptors (about 80%) above which extrapyramidal side effects (EPS) are likely to occur (105). In general, studies have failed to observe a relationship between the degree of D2 receptor occupancy and clinical response (106,107). However, most studies were performed at doses achieving more than 50% occupancy. Two studies performed with low doses of relatively selective D2 receptor antagonists (haloperidol and raclopride) suggested that 50% to 60% occupancy was required to observe a rapid clinical response (108,109). Clozapine, at clinically therapeutic doses, has been found to achieve only 40% to 60% D2 receptor occupancy (105,107,110), which, in conjunction with its anticholinergic properties, may account for its low risk for EPS. Occupancy of 5-HT2A receptors by “5-HT2A/D2 balanced antagonists” such as risperidone does not confer protection against EPS, since the threshold of D2 receptor occupancy associated with EPS is not markedly different between these drugs and drugs devoid of 5-HT2A antagonism (111,112,113,114). Studies with quetiapine suggest that, at least with this agent, transient high occupancy of D2 receptors might be sufficient to elicit a clinical response (115,116).
An interesting question relates to putative differences in the degree of occupancy achieved by antipsychotic drugs in striatal and extrastriatal areas. Pilowsky et al. (117) initially reported lower occupancy of striatal D2 receptors compared to temporal cortex D2 receptors in seven patients treated with the atypical antipsychotic drug clozapine, using the high affinity SPECT ligand [123I]-epidipride. In contrast, typical antipsychotics were reported to achieve similar occupancy in striatal and extrastriatal areas, as measured with [11C]-FLB457 (118) or [123I]-epidipride (119). It should be noted, however, that these very high affinity ligands do not allow accurate determination of D2 receptor availability in the striatum (120). Conversely, [18F]-fallypride enables accurate determination of D2 receptor availability in both striatal and extrastriatal areas (121). Occupancy studies using [18F]-fallypride confirmed that clozapine and quetiapine, but not olanzepine or haloperidol, achieved higher D2 receptor occupancy in temporal compared to striatal regions (122,123,124). Occupancy studies performed with [76Br]-FLB457 also reported higher occupancies in cortex compared to striatum for a number of antipsychotic drugs, including typical antipsychotic drugs (125). Conversely, a study combining [11C]-FLB457 imaging for extrastriatal D2 receptor receptors and [11C]-raclopride imaging for striatal D2 receptors suggested similar occupancy of D2 receptors in both regions for both typical and atypical antipsychotic drugs (126). Thus, at this point in time, there is a strong suggestion that many, if not most, antipsychotic drugs achieve higher occupancies in temporal cortex compared to striatum, although this phenomenon has not been universally observed. Factors underlying this difference remain to be elucidated.
Finally, it is important to point out that the most robust preclinical evidence relative to the site of therapeutic effect of antipsychotic drugs points toward the ventral striatum (65,127), while the imaging studies reviewed above contrasted striatal versus mesotemporal D2 receptor binding. Furthermore, D2 receptor occupancy levels in striatum have been shown to be more predictive of therapeutic response than in temporal cortex (128). Thus, the observation that, in a restricted dose range, D2 receptor occupancy by antipsychotic drugs is higher in temporal cortex than in striatum does not necessarily imply that the temporal cortex is the therapeutic site of actions of these agents.
Another unresolved question is the discrepancy in the value of D2 receptor occupancy obtained with [11C]-raclopride versus [11C]-NMSP. The haloperidol plasma concentration associated with 50% inhibition of [11C]-NMSP binding (3 to 5 mg/mL) (129) is ten times higher than that associated with 50% inhibition of [11C]-raclopride binding (0.32 ng/mL) (130). Quetiapine, at a dose of 750 mg, decreased [11C]-raclopride specific binding by 51%, but failed to affect [11C]-NMSP specific binding (131). These observations contribute to the debate regarding differences between benzamide and butyrophenone binding to D2 receptors.
Affective Disorders
Numerous abnormalities of regional cerebral blood flow (rCBF) and metabolism (rCMRglu) have been demonstrated in affective disorders using SPECT and PET. These studies have implicated anatomical circuits involving subregions of prefrontal cortex, striatum, amygdala, and hippocampus in the pathophysiology of these disorders (132,133). From a neurochemical perspective, abnormalities in several neurotransmitter systems may be relevant to the pathophysiology of depression. The 5-HT system has been the most extensively implicated, in part because of the antidepressant effect of medications that inhibit the synaptic reuptake of serotonin, as well as a wealth of postmortem, preclinical, and clinical data suggesting that reduced serotonergic function may be associated with depression (134). The recent availability of suitable PET radioligands for 5-HT2A receptors, 5-HT1A receptors, and SERT has allowed the in vivo investigation of their putative abnormalities in depression. In addition, a number of studies have evaluated potential alterations in DA systems in major depression.
This relatively recent literature is characterized by conflicting and inconsistent results, presumably related to the heterogeneity of major depression and to the relatively low number of subjects included in most studies. Effects of the clinical presentation (unipolar versus bipolar), clinical status (in episode versus in recovery), and medications have not yet been fully explored. Nonetheless, these studies have suggested that depression might be associated with lower SERT availability in the midbrain and lower 5-HT1A receptor availability in various limbic and cortical regions. No consistent pattern of alterations has yet emerged from the study of DA parameters in depression.
Major Depressive Disorder
Serotonin (5-HT) Transmission
5-HT2 Receptors
The earliest PET study of 5-HT2 receptors and depressive symptoms used [11C]-N-methyl-spiperone to investigate binding in patients with poststroke depression and reported increased binding (135). Yet, it is not clear how this finding can be generalized to more common clinical presentations of depression. Another early study using 2-[123I]-ketanserin and SPECT reported increased and asymmetrical cortical uptake of the tracer in depressed patients when compared to controls (136). However, 2-[123I]-ketanserin has significant limitations due to high nonspecific binding.
Since then, several PET studies have used newer 5-HT2 PET radiotracers, [18F]-setoperone (137) and [18F]-altanserin (138), to
investigate cortical 5-HT2A receptor binding in drug-free depressed patients. Biver et al. (139), using [18F]-altanserin, reported reduced tracer uptake in a region of the right hemisphere including the orbitofrontal cortex and the anterior insular cortex. Two studies investigated midlife depression using [18F]-setoperone and concluded that there is no major change or asymmetry in 5-HT2A receptors (140,141). In both studies the great majority of patients had been free of antidepressant medication for over 6 months. One study supported these negative findings and reported no significant alteration in 5-HT2A receptor binding in an untreated group of patients with late-life depression without cognitive impairment (142). Conversely, decreased 5-HT2A receptor binding was reported in the hippocampus of subjects suffering from late-life depression (143). Finally, the largest study to date (144) found a widespread reduction in 5-HT2A receptor binding potential (BP) and concluded that brain 5-HT2A receptors are decreased in patients with major depression. However, 40% of the patients in this study had been drug free for only 2 weeks before scanning. This factor may be significant as the majority of antidepressants down-regulate 5-HT2A receptors (141,145,146).
investigate cortical 5-HT2A receptor binding in drug-free depressed patients. Biver et al. (139), using [18F]-altanserin, reported reduced tracer uptake in a region of the right hemisphere including the orbitofrontal cortex and the anterior insular cortex. Two studies investigated midlife depression using [18F]-setoperone and concluded that there is no major change or asymmetry in 5-HT2A receptors (140,141). In both studies the great majority of patients had been free of antidepressant medication for over 6 months. One study supported these negative findings and reported no significant alteration in 5-HT2A receptor binding in an untreated group of patients with late-life depression without cognitive impairment (142). Conversely, decreased 5-HT2A receptor binding was reported in the hippocampus of subjects suffering from late-life depression (143). Finally, the largest study to date (144) found a widespread reduction in 5-HT2A receptor binding potential (BP) and concluded that brain 5-HT2A receptors are decreased in patients with major depression. However, 40% of the patients in this study had been drug free for only 2 weeks before scanning. This factor may be significant as the majority of antidepressants down-regulate 5-HT2A receptors (141,145,146).
In summary, three studies reported no significant alteration in 5-HT2A receptor binding in major depression, and three studies found reduced 5-HT2A receptors. Differences between studies might stem from methodological issues, illness heterogeneity, and medication effects. None of the recent studies confirmed the earlier findings of increased binding (135,136). Similarly, the increase in 5-HT2A receptors found in some, but not all, postmortem studies of suicide depressed victims (for review see Mann [147]) has not been confirmed by in vivo investigations. Therefore, there is currently no strong evidence supporting the hypothesis that depression per se is associated with marked alterations of 5-HT2A receptor density.
5-HT1A Receptors
Two lines of evidence have implicated the 5-HT1A receptors in depression. The first is the finding that depressed patients have blunted neuroendocrine responses to 5-HT1A receptor agonists in vivo, and the second is the dense distribution of these receptors in the hippocampus. Recent theories have implicated interactions between stress, corticosteroids, growth factors, and hippocampal 5-HT1A receptors in depression (148,149,150). Postmortem studies of 5-HT1A receptors in suicide and depression have been inconsistent, showing increased, decreased, and unchanged 5-HT1A receptors levels in various regions (151,152,153,154,155). These discrepancies may reflect the possible confounding effects of suicidality, antemortem medications, differences between radioligands, and differences in the regulation of 5-HT1A receptors by corticosteroids and local levels of 5-HT in different brain regions. The results of in vivo PET imaging of 5-HT1A receptors in depressed patients are therefore of interest.
Several PET studies have investigated 5-HT1A receptors in unmedicated depressed subjects using [11C]-WAY100635. The first study (156) found modest (approximately 10%) but significant widespread reductions in 5-HT1A receptor availability in cortical regions including medial temporal cortex (hippocampus and amygdala) in men with major depression. The same group reported a similar finding in patients with a history of major depression who were scanned after clinical recovery (157). Drevets et al. (148) reported comparable findings: reductions in the medial temporal cortex (27%) and raphe (41%). This group of subjects included both unipolar and bipolar depressed patients. The finding of lower raphe 5-HT1A receptor availability in depression was confirmed in a group of subjects with late-life depression (158). However, a study carried out in a large sample of subjects with major depressive disorder reported the opposite finding, an increase in 5-HT1A receptor availability in untreated subjects with major depression and no changes in treated patients (159). Higher baseline 5-HT1A receptor availability was associated with poorer response to antidepressant treatment (160).
Additional studies are warranted to further clarify the association between altered 5-HT1A receptor expression and depression. Since a major depressive episode is associated with hyperactivity of the hypothalamic-pituitary-adrenal axis, and increased cortisol levels might be associated with 5-HT1A receptor down-regulation (161,162,163,164), reduced 5-HT1A receptor availability might be secondary to the neuroendocrine dysregulation associated with depression. However, a direct test of this hypothesis found that a single dose of hydrocortisone failed to affect 5-HT1A receptor availability in recovered depressed patients (165).
Serotonin Transporter
Reductions in SERT levels in depressed patients have been reported in numerous postmortem studies (for review, see Mann [147]). The first ligand used to image SERT in vivo was the SPECT radiotracer [123I]-β-CIT. β-CIT binds to both DAT and SERT with comparable affinity (Ki 1.4 and 2.4 nM for DAT and SERT, respectively) (166,167). The lack of DAT versus SERT selectivity is not a problem for measuring DAT in the striatum, as the density of SERT in striatum is much lower than that of DAT (167). However, in the midbrain, this proportion is reversed, and the β-CIT midbrain uptake mostly corresponds to SERT binding (168,169). Studies in nonhuman primates and humans have shown that, in the midbrain, [123I]-β-CIT is selectively displaced by administration of selective serotonin reuptake inhibitors (SSRIs), but not by DAT selective drugs (168,170). [123I]-β-CIT has been extensively used in clinical studies both for striatal DAT (62,171,172,173,174,175,176) and midbrain SERT evaluation (62,177,178,179).
In depression, findings from three SPECT studies using [123I]-β-CIT were in agreement with postmortem results. A reduction in SERT binding was found in the midbrain in patients with unipolar depression (177) and in patients with atypical or melancholic depression (180), and in thalamus-hypothalamus in depressed patients with seasonal affective disorder (181).
The development of more selective SERT radiotracers was required to investigate SERT density in other regions of the brain. The first selective PET radiotracer available to measure SERT in humans was [11C]-McN5652 (182). The usefulness of [11C]-McN5652 as a PET tracer for SERT was validated in primates (183) and humans (184,185,186). However, [11C]-McN5652 has many limitations, which include high nonspecific binding, low signal to noise ratio, and slow clearance from the brain, restricting the use of this ligand to regions with relatively high SERT densities, such as midbrain, thalamus, and striatum (185).
Using [11C]-McN5652, one study found increased SERT availability in thalamus and unchanged SERT availability in midbrain in patients with major depression (187). In contrast, a [11C]-McN5652 study including a larger number of subjects with major depression found reduced SERT availability in midbrain (188). A study conducted in patients with bipolar disorder with [11C]-McN5652 reported lower SERT availability in midbrain, thalamus, and striatal regions (189).
Compounds from the phenylamine class have emerged as the most useful PET and SPECT SERT imaging agents to date. [123I]-ADAM (2-((2-dimethylamino)methyl)phenyl)thio)-5-iodophenylamine
and [11C]-ADAM (190,191,192,193) were the first imaging agents reported from this class and are highly selective SERT SPECT and PET imaging agents. Studies conducted with [123I]-ADAM in depression reported decreased (194) or unchanged (195) midbrain SERT availability.
and [11C]-ADAM (190,191,192,193) were the first imaging agents reported from this class and are highly selective SERT SPECT and PET imaging agents. Studies conducted with [123I]-ADAM in depression reported decreased (194) or unchanged (195) midbrain SERT availability.
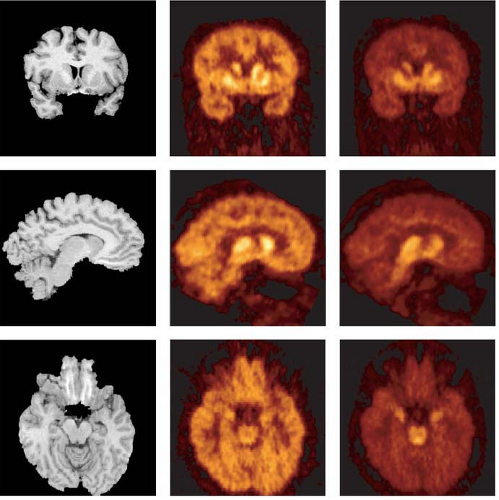
Another compound in this series, [11C]-DASB, was found to provide good imaging qualities for SERT quantification (196,197,198) and to be superior to [11C]-McN5652 in humans (199) (Fig. 10.2). Using [11C]-DASB, one study reported no changes in regional SERT availability in patients with major depressive episodes (200).
Together, studies of SERT availability suggest that depression might be associated with lower SERT availability in the midbrain, although this finding has not been reported in all studies. One should note that even the best SERT radiotracer reported to date, [11C]-DASB, is inadequate to measure SERT in regions with relatively low SERT density, such as the frontal cortex. Novel SERT imaging agents with higher signal-to-noise characteristics are actively being developed to fill this gap (see for example Huang et al. [201]).
Dopamine Transmission
The critical role of DA in brain reward systems, the reports of low cerebrospinal fluid homovanillic acid levels in depressed patients, the association of major depression with Parkinson disease, and the enhancement of dopaminergic activity by several antidepressant treatments suggest that a deficiency of dopaminergic function might be associated with major depression (for review see Kapur and Mann [202]) (203,204,205). Five studies compared striatal D2 receptor availability with [123I]-IBZM and SPECT in patients with major depression and control subjects. Two of five studies reported higher [123I]-IBZM specific binding in the striatum of depressed subjects compared to controls (206,207), whereas three studies reported no changes (52,208,209). Using [11C]-raclopride and PET, one study reported elevated D2 receptor availability in putamen in patients with depression with motor retardation (210). Amphetamine-induced DA release was also assessed in patients with major depression and found to be unchanged (52).
Two studies examined [123I]-β-CIT striatal binding to DAT in patients with major depression and yielded conflicting results: one study reported normal levels of striatal DAT in patients with major depression (177), while the other reported increased DAT levels (211). One study reported decreased DAT density in depression using [11C]-RTI32 (212). SPECT studies conducted with [99mTc]-TRODAT1 also reported conflicting results, with studies reporting increased (213) or unchanged (214) striatal [99mTc]-TRODAT1 uptake in patients with major depression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree