Pulmonary
The lungs have traditionally been considered a barrier to ultrasound imaging because large changes in acoustic impedance result in ultrasound reflection and the acoustic impedance of air is extremely low compared with anatomic tissues. It was not until 1986 that the diagnosis of pneumothorax with ultrasound was reported in veterinary medicine.1 In 1995, Lichtenstein published his landmark paper describing the most fundamental element of pulmonary ultrasound, the lung sliding sign.2
Lichtenstein opened the door for a large body of research based on the analysis of artifacts generated by the nearly complete reflection of the ultrasound beam when it encounters the interface between soft tissue and aerated parenchyma of the lung. What was initially seen as “noise” became useful information. The tissues and interfaces reflect the sound waves exhibiting notably different kinds of “noise” artifacts in several normal and pathologic conditions.
In 2011, the International Liaison Committee for the International Consensus Conference on Lung Ultrasound (ICC-LUS) critically evaluated the literature regarding point-of-care lung ultrasound. Over 300 publications were reviewed. From this, a consensus statement was written.3 Overwhelmingly, the recommendations support the use of ultrasound to evaluate the lungs in critically ill and injured patients.4–6
 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS
The chest radiograph historically is one of the most iconic elements of the practice of medicine. It is understandable that many physicians resist ultrasound for lung pathology given the ingrained role of radiography. The advantages of lung ultrasound, however, outweigh the challenges of learning a new practice.
Sonography has many advantages over plain films and cross-sectional imaging. It is highly portable allowing its use in situations of limited resources as well as austere conditions. Ultrasound is feasible at the bedside and improves interaction during clinical interview increasing patient satisfaction.7 Furthermore, the pulmonary applications of ultrasound consistently present levels of accuracy superior to plain films and comparable to CT scans, without exposing the patient to radiation.8–10
Point-of-care ultrasound provides an immediate diagnostic answer without the delays of media processing, transport, and consultative interpretation. This is particularly important in the critically ill.
Ultrasound plays a critical role for diagnosing lung pathology, including pneumothorax, hemothorax, interstitial syndromes, pneumonia, pulmonary edema, and contusion.3 Point-of-care ultrasound provides the clinician with immediate, accurate data regarding lung pathology.
 CLINICAL INDICATIONS
CLINICAL INDICATIONS
Pulmonary ultrasound should be considered a first-line diagnostic modality for critically ill patients.3 Point-of-care ultrasound, particularly lung ultrasound, is widely referred to as “the new stethoscope.”
Clinical scenarios for performing lung ultrasound include
 Evaluation of acute dyspnea
Evaluation of acute dyspnea
 Airway management
Airway management
 Pneumothorax
Pneumothorax
 Alveolar-interstitial syndromes: cardiogenic pulmonary edema, acute respiratory distress syndrome (ARDS), pulmonary contusion, and other interstitial syndromes
Alveolar-interstitial syndromes: cardiogenic pulmonary edema, acute respiratory distress syndrome (ARDS), pulmonary contusion, and other interstitial syndromes
 Consolidations: pneumonia, atelectasis, other nonpneumonic consolidations
Consolidations: pneumonia, atelectasis, other nonpneumonic consolidations
 Neonatal applications
Neonatal applications
 Pleural effusions
Pleural effusions
EVALUATION OF ACUTE DYSPNEA
Critical ultrasound achieves its prime role in the hands of the emergency and critical care physicians when dealing with unstable patients by providing immediate diagnostic answers, allowing for immediate therapeutic decisions. A number of ultrasound-guided protocols for diagnosis of undifferentiated shock rely heavily on chest ultrasound for the assessment of pneumothorax, pulmonary congestion, and pulmonary embolism.4,11–17 Lichtenstein published the BLUE (bedside lung ultrasound in emergency) protocol18 in 2008, which is primarily based on point-of-care lung ultrasound. This algorithm is a technically simple approach for the statistically accurate diagnosis of severe dyspnea in a selected population of intensive care patients. A similar approach was used in an ED population.19 A high concordance between ultrasound and chest radiography was reported, and it questioned whether point-of-care ultrasound could replace standard chest radiography for evaluation of acute dyspnea in the ED. According to the evidence-based recommendations from the ICC-LUS, sonography should be considered a primary imaging modality for the diagnosis of acute dyspnea.3
AIRWAY MANAGEMENT
Sonography has a promising role in invasive airway management, especially with ultrasound-guided cricothyrotomy and percutaneous tracheostomy. Emergency physicians can use ultrasound to reliably identify the cricothyroid membrane,20 but the clinical use is still to be investigated. Sonography assists with confirmation of endotracheal tube placement. Advantages over the traditional methods include detection of main stem intubation21–23 and freedom from the false-negative results presented by end-tidal CO2 detectors in hypoperfusion states.24 These applications will be discussed in detail in Chapter 8, “Critical Care,” and Chapter 22, “Additional Ultrasound-Guided Procedures.”
PNEUMOTHORAX
The most widely used application of lung ultrasound in the ED is the evaluation for pneumothorax in trauma. There is extensive literature supporting the superiority of ultrasound over plain films in this role.25–37 This technique is discussed in Chapter 5, “Trauma,” and Chapter 8, “Critical Care.”
ALVEOLAR-INTERSTITIAL SYNDROMES
Cardiogenic Pulmonary Edema
One of the most exciting applications of lung ultrasound is cardiogenic pulmonary edema. Acutely dyspneic patients present diagnostic dilemmas due to the overlap and frequent coexistence of pathology as well as the time-dependent need for diagnosis and intervention. Acutely decompensated heart failure (ADHF) presents with the classic acutely dyspneic patient. Traditional ancillary testing, such as chest radiograph and labs, provides only moderate increase in our diagnostic accuracy and invokes tremendous delay in diagnosis.38–40
Sonography is a powerful tool to differentiate ADHF from other clinical entities,4,41–46 and has been shown to provide quantitative information about the degree of pulmonary edema.46–48
ADHF presents a very typical finding on the ultrasound examination, called the B-pattern.49 This pattern is based on the finding of multiple B-lines throughout the entire pulmonary surface. B-lines are the artifact found in the alveolar-interstitial syndromes and are generated when there is alveolar flooding and/or thickening of the interstitium by extravascular lung water or inflammatory products. In an ICU population, 100% of patients with ADHF had diffuse B-lines on exam, while 92% of COPD exacerbations did not.50
In a study of dialysis patients, a linear correlation was found between removed fluid and decrease in the quantity of B-lines.48 The study confirmed previous findings that B-lines are present earlier than the clinical symptom of dyspnea is perceived by the patients.47 The appearance of B-lines prior to physiologic dysfunction was also found in high-altitude pulmonary edema51 and ARDS.52
B-lines (previously called “comet tails”) are defined as discrete “laser-like vertical hyperechoic reverberation artifacts that arise from the pleural line, extend to the bottom of the screen without fading, and move synchronously with lung sliding.”3 (Figure 7-1). The diagnosis of ADHF can usually be made within seconds in a patient with a consistent history.39
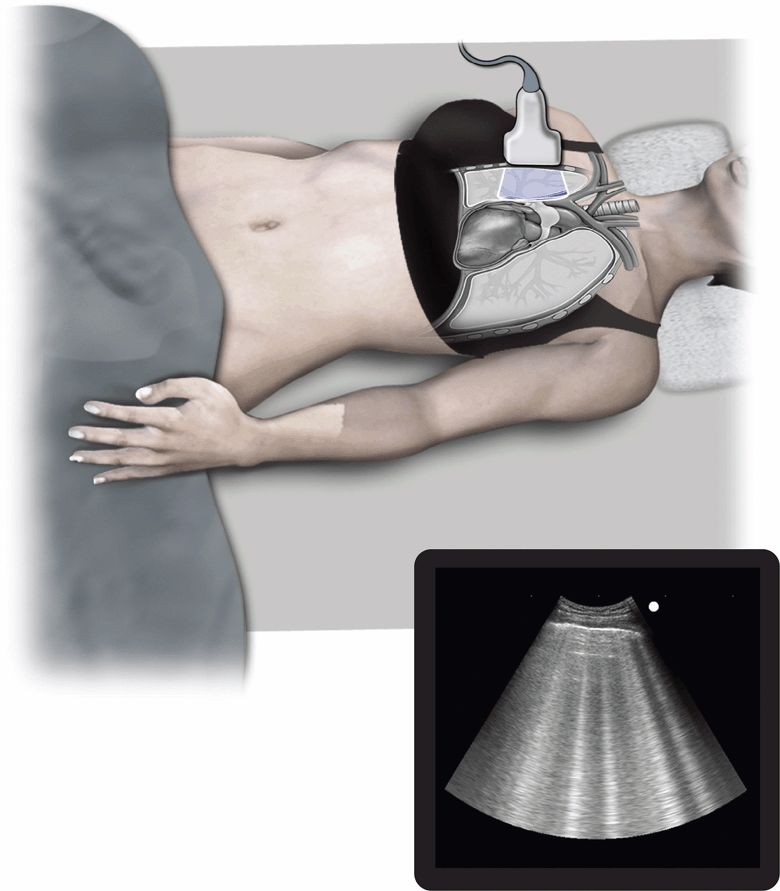
Figure 7-1. Pulmonary edema. Ultrasound techniques and findings are outlined in the corresponding sections of this chapter.
Often, scanning one focal area on the anterior chest at each hemithorax in a dyspneic patient is enough to assess for pulmonary congestion.18 Even in cases where the clinical history is not immediately conducive to the diagnosis of ADHF, lung ultrasound still is recommended as a first-line diagnostic tool, together with the traditional methods.3
ARDS and Other Interstitial Syndromes
ARDS can be clinically indistinguishable from ADHF, and there are several other diseases that mimic its clinical and radiologic appearance.53 ARDS is still a diagnosis of exclusion, and the first step is excluding cardiogenic pulmonary edema.54 BNP levels are a widely available test, but unfortunately are useful only if below 100 pg/mL, when they are suggestive of a noncardiogenic process. Levels above 100 pg/mL cannot confirm heart failure nor exclude ARDS.55,56
All interstitial syndromes that involve the subpleural parenchyma present a similar finding on lung ultrasound (B-line pattern). However, the findings of pleural line abnormalities (irregular, thickened, fragmented), anterior subpleural consolidations, presence of spared areas, reduction or absence of lung sliding, and nonhomogenous distribution of B-lines distinguish ARDS from ADHF.3
One review suggested that ultrasound could completely replace plain films and even CT scan for the diagnosis and monitoring of patients with ARDS and its usual complications, such as effusion and pneumothorax.57 The same group reported successful use of ultrasound for guidance of alveolar recruitment maneuvers in ARDS.58
Pulmonary ultrasound has been shown to be useful in the diagnosis of other interstitial processes, like pulmonary contusion,59–61 acute chest syndrome,62 pulmonary fibrosis,63–65 and viral pneumonitis.66
CONSOLIDATIONS
Several disease processes cause consolidation of the lung parenchyma. These include pneumonia, atelectasis, infarction (pulmonary embolism), and malignancy. Lung consolidation has a very characteristic ultrasound appearance; however, there are also some findings that allow differentiation between consolidative processes.3,18,67
Pneumonia
Clinical signs68 and symptoms of pneumonia have a sensitivity of less than 50% compared with chest radiograph.69 Although chest radiography is the most common way to diagnose pneumonia,70 it has a sensitivity under 75,71–74 compared with CT. Although CT is extremely sensitive for the diagnosis of pneumonia, it is not appropriate for routine use.70 The literature supports using ultrasound for the diagnosis of pneumonia, with a reported sensitivity between 89% and 97% and a specificity between 95% and 98%.3,8,9,75–81 In addition to improved sensitivity, point-of-care ultrasound allows clinicians to perform serial exams in order to look for disease progression or ensure resolution of pneumonia without additional radiographic exams.8–10
Pulmonary consolidations that extend to the pleura are generally well visualized with ultrasound. One study showed that the consolidation reached the pleura in 98.5% of critically ill patients with CT-proven lung consolidations.82 Limited data suggest that the sensitivity of lung ultrasound for the diagnosis of pneumonia in the ED may be better than the ICU setting,75,82 possibly due to fewer limitations with patient mobility, dressings, tubes, etc.
The idea of imaging the entire pulmonary surface for consolidations may seem daunting to a time-pressed emergency physician; however, this examination may be successfully completed in less than 5 minutes.75,77
Nonpneumonic Subpleural Consolidations
Sonographic findings can reliably differentiate other causes of lung consolidation, particularly when providers use a combination of clinical and ultrasound findings to make a diagnosis.4,24,61 Ultrasound findings that help define different types of consolidation are shape, boundaries, the presence and morphology of internal echoes (bronchograms), adjacent artifacts, and vascularity.3,9,67,80–84 In addition, contrast agents are very useful for the evaluation of lung consolidations because different disease processes demonstrate remarkably different patterns of vascular morphology.
Pulmonary embolism is the most controversial topic in the lung ultrasound literature. Findings compatible with pulmonary embolism are wildly dichotomous. The literature espouses both a normal lung exam and pulmonary infarctions as findings consistent with pulmonary embolism. In the literature originating from critical care populations, the key finding in pulmonary embolism is a normal lung examination in the setting of DVT.18 In contrast, other studies on nonacute and critically ill patients show that the vast majority presented with ultrasound findings compatible with pulmonary infarctions.67,85,86 In this population, the overall accuracy of the examination was also increased if DVT was found. Our interpretation of this difference in the literature is that critical patients with massive or submassive pulmonary embolisms may have fewer but larger emboli, located more centrally in the vasculature, and with shorter time to examination. The noncritical patients may have a larger number of smaller emboli, with longer time before examination allowing for the complete development of the parenchymal inflammatory process and peripheral infarctions. Furthermore, unstable patients have had most of their examinations limited to the anterior chest, while most emboli are posterior.87–89
NEONATAL APPLICATIONS
Pulmonary ultrasound is revolutionary for the evaluation of respiratory distress in the neonatal population. It is remarkably accurate and negates radiation exposure in this sensitive group.90,91 The cartilaginous ribcage and the small size of the lungs of a newborn allow for better evaluation and a complete exam.
Lung ultrasound was found to be useful for the diagnosis of transient tachypnea of the newborn.92 In a mixed population of 132 infants with normal lungs or multiple interstitial diseases [transient tachypnea of the newborn, neonatal respiratory distress syndrome (NRDS), alveolar hemorrhage, pneumonia, atelectasis], a sensitivity and specificity of 100% compared with chest radiography were found.
Another study of 40 premature infants with radiographic signs of NRDS found ultrasound to be 100% sensitive and 100% specific,93 suggesting that it could replace chest radiography, similar to what has been shown in adults with ARDS.94,95
PLEURAL EFFUSIONS
Ultrasound has a well-established role for the diagnosis and treatment of pleural effusions.96 In addition to the intrinsic advantages of sonography over other methods, it is far more sensitive than plain films97 and allows for differentiation of physiologic effusions,98 and exudative and transudative processes.99 In addition, ultrasound can differentiate effusion from pleural thickening,100 estimate effusion volume in sitting or supine patients,101 and drastically increase the safety102 and success rates103 of thoracentesis. Ultrasound-guided thoracentesis is covered in Chapter 22, “Additional Ultrasound-Guided Procedures.”
 ANATOMIC CONSIDERATIONS
ANATOMIC CONSIDERATIONS
The subunits of the right and left lungs are called segments. The right lung is comprised of 10 segments: 3 in the right upper lobe (apical, anterior and medial), 2 in the right middle lobe (medial and lateral), and 5 in the right lower lobe (superior, medial, anterior, lateral, and posterior). The left lung comprises 8 segments: 4 in the left upper lobe (apicoposterior, anterior, superior lingula, and inferior lingula) and 4 in the left lower lobe (superior, anteromedial, lateral, and posterior). The lungs are covered by the visceral pleura, which is contiguous with the parietal pleura as it reflects from the lateral surfaces of the mediastinum.
Ultrasound of the lungs depends on findings generated by sonographic reflection from the lung and pleura. The lungs have a very large surface area, not all of which is accessible to ultrasound. Paravertebral regions and areas under the shoulders are not visible. Individual patient characteristics may limit the examination. Most pulmonary disease processes present with primary or secondary sonographic signs. They often involve a large portion of the pulmonary surface and/or accumulation of fluid in dependent regions. The combination of primary and secondary findings increases the sensitivity of pulmonary ultrasound.
In a group of 260 critically ill dyspneic patients, the correct diagnosis was reported in 53% of the patients by using a limited ultrasound exam that concentrated mostly on the anterior chest wall.88 All examinations were performed in less than 3 minutes. The patient who presents with undifferentiated dyspnea will likely be in a semirecumbent or sitting position. The examination of a single spot104 on each side of the anterior chest will likely provide the diagnostic answer because ADHF usually demonstrates diffuse B-lines bilaterally. However, routinely including an exam of the lateral chest may significantly increase sensitivity.105
 GETTING STARTED
GETTING STARTED
BASIC PRINCIPLES OF LUNG ULTRASOUND
Pulmonary ultrasound is different from other sonographic examinations in that artifacts are good. Most lung ultrasound findings are based on the analysis of the artifacts, not of anatomical images. When encountering the interface of soft tissue and air at the level of the pleural line, the ultrasound beam is almost completely reflected back to the transducer. Think of air for ultrasound as a mirror is for light. This will help with the understanding of how both normal and pathologic pulmonary artifacts are generated.
MACHINES, TRANSDUCERS, AND SETTINGS
Less image processing is better since the goal in lung ultrasound is analysis of artifacts. Disable advanced filters, particularly tissue harmonics and spatial compounding. These capabilities were developed to eliminate the artifacts that decrease image quality for anatomical images.These artifacts, however, are essential for pulmonary sonography.
Many contemporary machines do not have a factory preset for lung imaging. Nevertheless, disabling filters usually allows adequate imaging of the normal and pathologic lung artifacts.
If creation of a lung-specific preset is desirable, several settings have been found to be useful. Begin with a value of zero for the setting “persistence” and a high-contrast gray scale map. The other adjustments should favor achieving high frame-per-second rates, like the cardiac presets, because of the constant movement intrinsic to the pulmonary artifacts.
In spite of the enthusiastic recommendation of some authors for specific transducers or machines, there is no consensus among the experts.3 Simple gray scale-only machines and microconvex 5 MHz transducers were traditionally recommended, but successful and comparable studies have been performed with virtually all common transducer arrays and machines. More superficial structures (e.g., pleura and chest wall) are better examined with high-frequency linear transducers to image anatomic detail. Deeper ultrasound-conducting structures (e.g., pleural effusions and consolidations) are better identified with lower frequency curvilinear or phased array transducers.
Our general recommendation considers clinical setting and goals of the examination. In the complete initial assessment involving an unstable or acutely ill patient, where multiple clinical questions exist, ideally the same transducer should be used for the entire examination. In this scenario, use phased array or curvilinear transducers. Examples of this include the acutely dyspneic patient, the critically injured patient, and the undifferentiated hypotensive patient; this helps avoid delays when switching transducers. To evaluate a focal process in a nonemergent fashion, such as detailed analysis of a subpleural consolidation, linear transducers afford superior resolution. An example would be differentiation of a metastatic lesion from a pulmonary infarct.
 TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
LUNG SLIDING SIGN
The interface of the parietal and visceral pleura is the first element to consider when performing a pulmonary ultrasound exam. It is the initial reference for all normal and pathologic findings. The lung sliding sign is visible as a hyperechoic horizontal line at the pleural level, immediately deep to the ribs, which presents to-and-fro movement with ventilation. It is generated by the reflection of the ultrasound beam off the air contained in the aerated parenchyma, and the movement represents the visceral pleura sliding against the parietal pleura (See Video 7-1: Thoracic Normal).
Correctly locate the level of the pleural line when assessing lung sliding. Start the lung exam with a vertical transducer orientation in order to visualize at least two ribs as reference points so that the position of the pleural line can be identified with certainty as it extends between them (Figure 7-2).
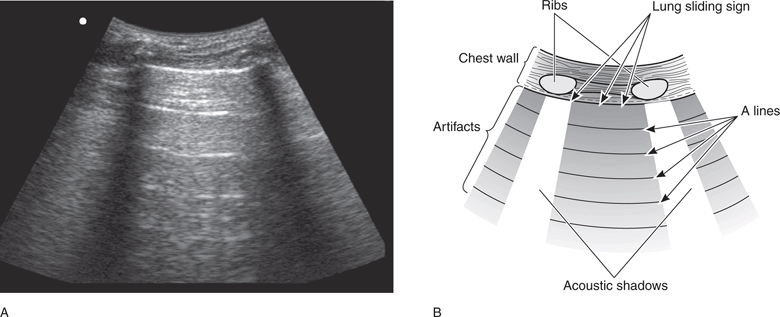
Figure 7-2. Normal lung. (A) Longitudinal intercostal scan. (B) Schematic representation of the same sonographic image.
Once the pleural line is identified, change the transducer orientation to remove the ribs from view and scan a larger length of pulmonary surface through the intercostal space.
When a pneumothorax is suspected, begin the exam on the anterior portion of the chest with the patient in a supine position (See Video 7-2: Thoracic Abnormal). Free air will accumulate in the least dependent region of the chest cavity. If the initial exam shows lung sliding, then no further scanning is needed. If lung sliding is absent, then a pneumothorax is most likely present, but a more detailed exam can confirm the diagnosis. In this case, move the transducer laterally to find the spot where the lung loses contact from the chest wall—the lung point33—which clinches the diagnosis.
M-MODE SIGNS
The spherical shape of the alveoli gives the normal pleural interface a “rough” sonographic aspect by reflecting the ultrasound waves in multiple directions and causes the artifact below to present a “sparkling” pattern. The examination of normal lung in M-mode generates a pattern called the “seashore sign” (Figure 7-3A). This is not seen in the presence of a pneumothorax since the ultrasound beam is reflected off the smooth layer of air contained within the parietal pleura; hence, lung sliding sign is absent and the artifact is static. M-mode generates the linear pattern called the “stratosphere sign” (Figure 7-3B), which suggests the presence of a pneumothorax.
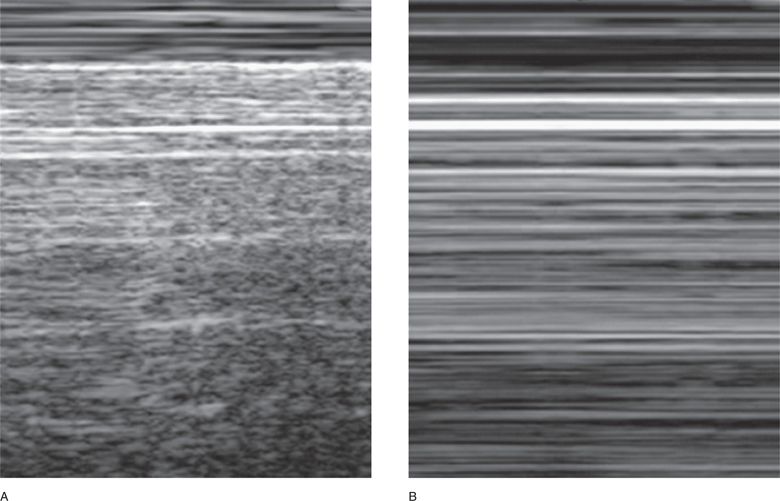
Figure 7-3. M-mode signs. (A) The seashore sign: note the two distinct patterns. The more superficial linear pattern is the tracing of the chest wall, devoid of any movement (the “waves”), and the deeper grainy pattern generated by the tracing of the sparkling artifact during normal pleural movement (the “sandy beach”). (B) The stratosphere sign: only horizontal lines are traced, recording the complete absence of movement on the screen. Both the chest wall and the artifact are static.
A-LINES
When the ultrasound beam reaches the pleural line, it is reflected almost entirely by the air in the alveoli or by a layer of free air. This causes a reverberation of the ultrasound waves within the thickness of the chest wall and the machine produces this image an infinite number of times. The image generated is expressed as the artifact below the pleural line as multiple horizontal lines (repetitions of the pleural line) separated by the distance equivalent to the thickness of the chest wall. These are called A-lines. They are seen whenever there is aerated parenchyma (e.g., normal lung, central pulmonary embolism, and COPD) or free air (pneumothorax). Alines are more easily generated by free air than by aerated parenchyma. Alines are usually present in images of normal lungs and are usually absent in the setting of interstitial syndromes, consolidations, or effusions.
 COMMON AND EMERGENT ABNORMALITIES
COMMON AND EMERGENT ABNORMALITIES
Understanding how lung ultrasound artifacts are generated makes it easier to appreciate how normal and pathologic images relate to specific disease processes. There is a predictable progression in the pattern of artifacts as the water/air ratio increases (Figure 7-4).37
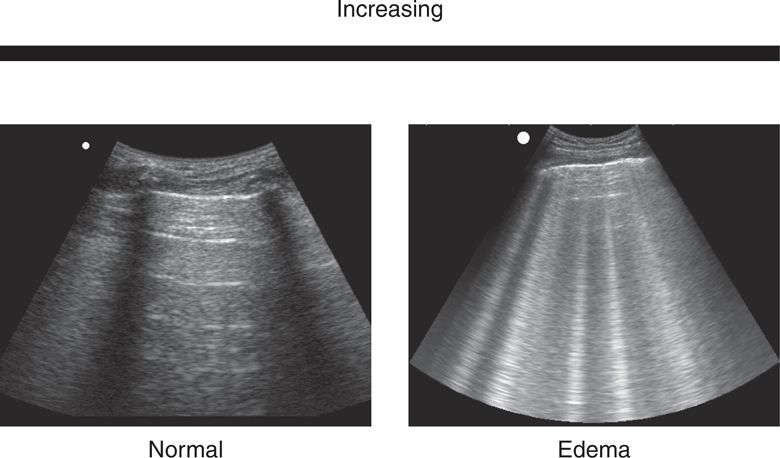
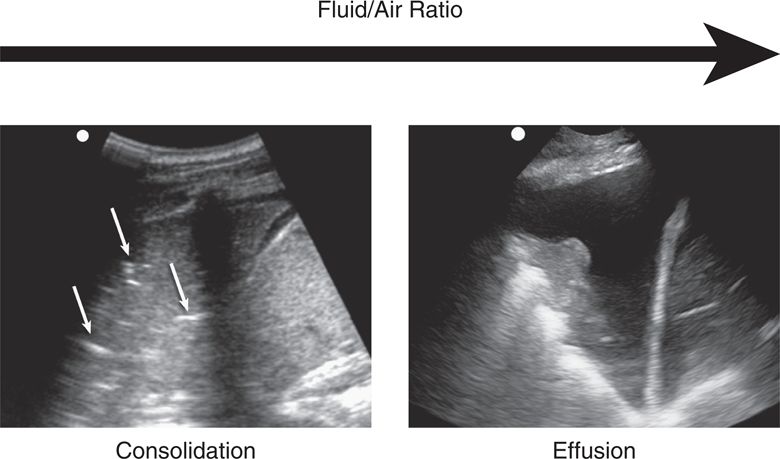
Figure 7-4. Progression of the artifacts in lung ultrasound according to increase in fluid/air ratio.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS CLINICAL INDICATIONS
CLINICAL INDICATIONS ANATOMIC CONSIDERATIONS
ANATOMIC CONSIDERATIONS GETTING STARTED
GETTING STARTED TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS COMMON AND EMERGENT ABNORMALITIES
COMMON AND EMERGENT ABNORMALITIES PITFALLS
PITFALLS CASE STUDIES
CASE STUDIES