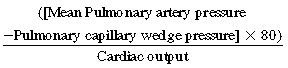CHAPTER 14 Pulmonary and bronchial arteries
Arteriography
Pulmonary arteriography (video 14-1)
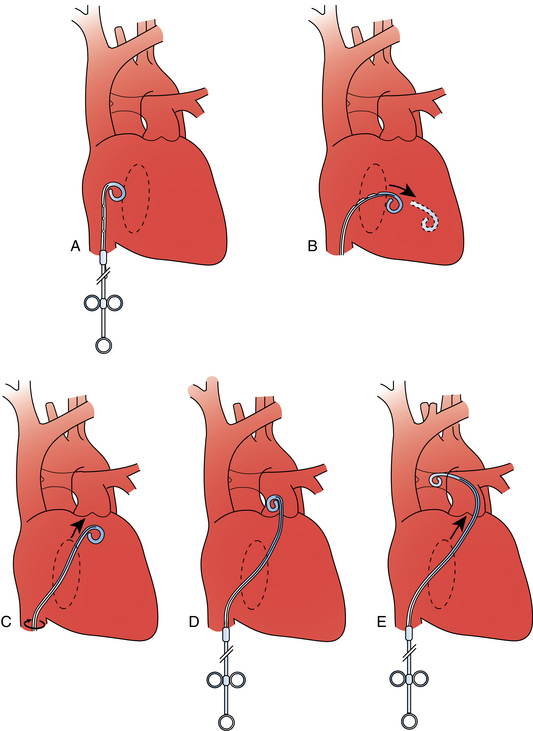
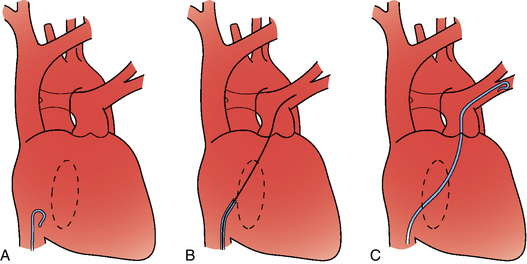
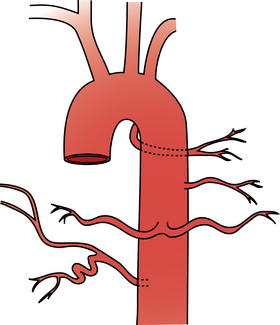
Figure 14-3 Pulmonary arteriography using a pigtail catheter and deflecting wire. A, The pigtail is advanced to the right atrium. B, While a bend is placed on the tip-deflecting wire, the catheter is fed off into the right ventricle. C, The deflection is released, straightening the catheter. D, The catheter is rotated and advanced through the right ventricular outflow tract into the main pulmonary artery. E, The catheter can be directed into the right or left pulmonary artery by rotating the pigtail toward the desired side and feeding it off the deflected wire.
(Adapted from Kadir S. Diagnostic angiography. Philadelphia: WB Saunders, 1986:590.)
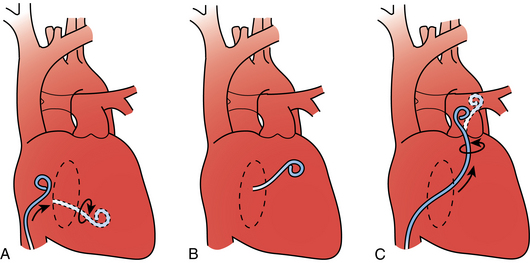
Extreme care should be taken while traversing the right heart chambers. Short bursts of atrial or ventricular tachycardia almost always stop by quickly repositioning the catheter or guidewire. For sustained or recurrent ventricular tachycardia, amiodarone (150 mg intravenously over 10 minutes) may be required. Cardiac perforation is avoided by gentle catheter manipulation. Inability to advance the catheter from the right atrium to the right ventricle may result from inadvertent entry into the coronary sinus (Fig. 14-4).
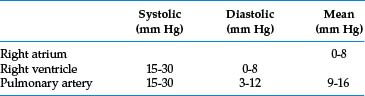
Pulmonary artery pressures are obtained routinely before contrast injection. Table 14-1 lists normal right heart pressures.4 The pulmonary artery pressure may be elevated for a variety of reasons5 (Box 14-1). In patients with high right heart pressures, it is prudent to limit each contrast injection to about 20 mL. Interventionalists have personal preferences for tube angulation for pulmonary arteriography. In general, lower lobe vessels are displayed best in the ipsilateral posterior oblique projection.
Box 14-1 Causes of Pulmonary Artery Hypertension
From Simonneau G, Galie N, Rubin LJ, et al: Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43 Suppl 1:5S-12S.
Adverse events include contrast reaction, transient renal dysfunction, access site hematoma, dysrhythmias, and respiratory distress. In older series, the risks of minor complications, major complications, and death resulting from pulmonary angiography were about 5%, 1%, and 0.2 to 0.5%, respectively.6 There are conflicting data regarding the increased risk of pulmonary arteriography in patients with pulmonary artery hypertension.4,5 However, in one recent report, all fatal complications (1.5%) occurred in patients with acute pulmonary artery hypertension.7 Cardiac perforation is exceedingly rare when appropriate catheters are used.
Bronchial arteriography
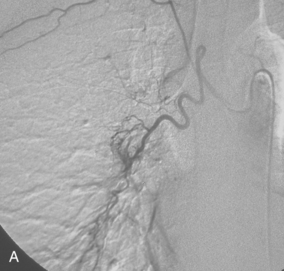
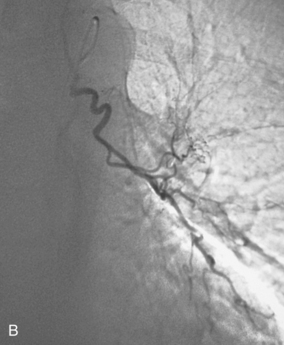
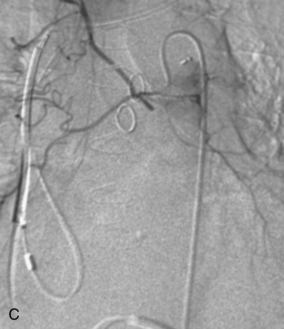
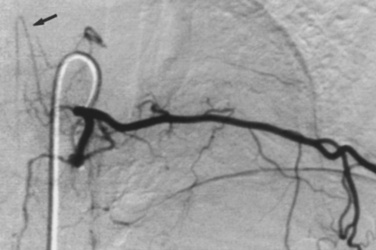
Selective catheterization of the bronchial arteries can be performed with forward-seeking catheters (e.g., spinal or renal double curve catheter) or reverse-curve catheters (e.g., Simmons or Shetty). In any case, the catheter tip must be wall-seeking along the descending thoracic aorta to engage the bronchial arteries. Forward-seeking catheters are easier to maneuver but may be more difficult to seat within the target vessel. Reverse-curve catheters can be more difficult to manipulate, particularly near the aortic arch, but they engage the artery more securely. The bronchial arteries are found by scraping the catheter tip along the aortic wall in the anticipated location of the vessels (level of the fourth to sixth thoracic vertebrae), which corresponds to the position of the left main stem bronchus (Fig. 14-5). A descending thoracic aortogram may be obtained to identify bronchial artery origins if they cannot be catheterized easily. High-quality images are critical to identify small bronchial artery branches that may supply the anterior spinal artery.
Anatomy
Development
Early in fetal development, the lung buds are supplied by a network of vessels arising from the aortic sac, which itself is composed of the paired ventral aortae.8 The sixth aortic arch becomes the conduit between the pulmonary trunk (which arises from the aortic sac) and the right and left pulmonary arteries (see Fig. 6-1). The ventral segment of the right sixth aortic arch becomes the right pulmonary artery origin. The ventral segment of the left sixth aortic arch develops into part of the pulmonary trunk, and the dorsal segment becomes the ductus arteriosus.
Normal pulmonary vascular anatomy and physiology
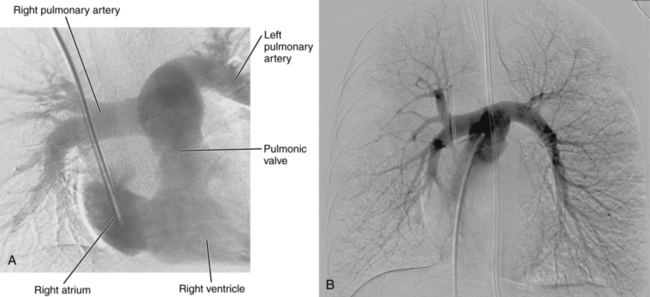
The pulmonary artery arises from the base of the right ventricle.9 It courses superiorly to the left of the ascending aorta and then divides below the aortic arch into right and left pulmonary arteries (Fig. 14-6). The bifurcation is located inferior, anterior, and to the left of the tracheal bifurcation.
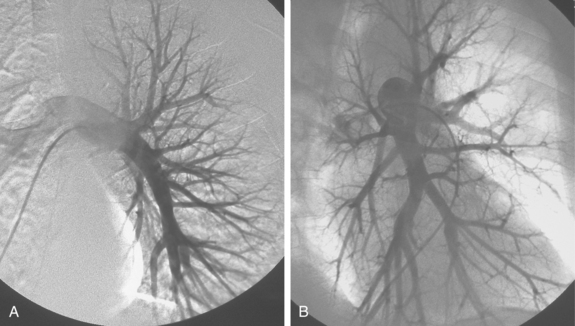
The right pulmonary artery runs horizontally behind the ascending aorta and superior vena cava (SVC) and in front of the tracheal bifurcation and esophagus to reach the hilum of the right lung (Fig. 14-7). It divides behind the SVC into an ascending branch (truncus anterior) supplying the right upper lobe and the descending right pulmonary artery supplying the middle and lower lobes. The ascending trunk usually has three branches to the segments of the right upper lobe: apical, posterior, and anterior. Occasionally, two segmental branches arise in a common trunk. The descending right pulmonary artery courses behind the intermediate bronchus. This artery commonly gives off an accessory branch to some portion of the right upper lobe. The descending pulmonary artery then provides an anterior branch supplying the right middle lobe and a posterior branch supplying the superior segment of the right lower lobe. The descending pulmonary artery continues inferiorly and then divides. Although the branching pattern is somewhat variable, the relative positions of the segmental branches is fairly predictable on a frontal pulmonary arteriogram going from lateral to medial: anterior, lateral, posterior, medial.
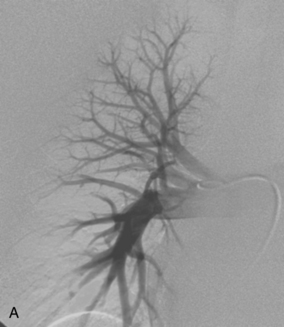
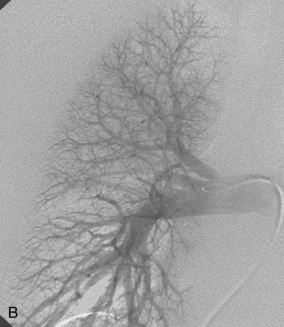
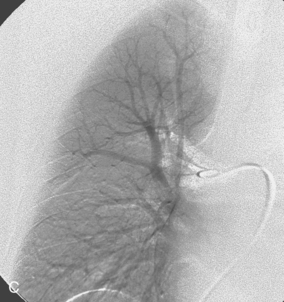
Figure 14-7 Normal right pulmonary arteriogram. A, Early arterial phase. B, Late arterial phase. C, Venous phase.
The left pulmonary artery courses upward and posteriorly in front of the aorta and the left main stem bronchus (Fig. 14-8). The ascending branch of the left pulmonary artery divides into apical posterior and anterior segmental arteries. The first one or two branches of the descending left pulmonary artery are the superior and inferior segmental arteries, which supply the lingula. The left pulmonary artery then divides into several vessels supplying the segments of the left lower lobe. The relative location of the terminal portions of the left lower lobe vessels is the mirror image of the pattern just described for the right lung.
Because the venous drainage of the lungs is located within the interlobular septa, the pulmonary veins do not follow the pulmonary arteries and bronchi.10 The right and left lungs are drained by superior and inferior pulmonary veins (Fig. 14-9). On each side, the vessels usually enter the left atrium separately, inferior to the pulmonary arteries. In some cases, they form a confluence before draining into the left atrium. The superior pulmonary veins drain the upper and middle (or lingular) lobes of each lung. The inferior pulmonary veins drain the lower lobes.
The pulmonary circulation is a high-flow, low-pressure system with a total resistance about one eighth to one tenth that of the systemic circulation.11Pulmonary vascular resistance (PVR) is calculated as
The normal range is 90 to 250 dyne/sec/cm, which in Wood units (PVR/80) is 0.7 to 1.1. While standing, blood flows preferentially to the lower lobes. When supine, blood flow is more evenly distributed between the lung bases and apices. Because the system is so distensible and has a large number of reserve vessels, sudden obstruction of one half of the pulmonary circulation in a patient without preexisting cardiopulmonary disease only slightly elevates pulmonary artery pressure. However, in patients with preexisting cardiac or pulmonary disease, which may be associated with elevated right heart pressures and right ventricular dysfunction, acute obstruction of even a small portion of the pulmonary circulation may cause a significant rise in pulmonary artery pressure.
Normal bronchial artery anatomy
The bronchial arteries supply the trachea, bronchi, esophagus, and posterior mediastinum, and they also act as the vasa vasorum of the pulmonary arteries. These vessels may not be visible at thoracic aortography in patients without lung disease. However, they become markedly enlarged and serve as an important collateral pathway in patients with certain congenital heart diseases, chronic lung infections, lung tumors, and obstruction of pulmonary arteries or veins.12 Bronchial artery anatomy is extremely variable.13–15 Anatomic and angiographic studies differ in the reported pattern and distribution of their origins. As a rule, one or two bronchial arteries supply each lung; however, up to four arteries may supply one side. Several patterns are commonly observed (Fig. 14-10; see also Fig. 14-5):
Rarely, a right intercostal-bronchial artery trunk gives rise to a left bronchial artery. Unusual bronchial artery origins are noted in Box 14-2.
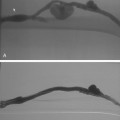
Bronchial arteries may have branches to the anterior spinal artery, which supplies the ventral portion of the spinal cord. In a few individuals, a right bronchointercostal artery (and rarely a right or left bronchial artery) feeds the great anterior radicular artery (artery of Adamkiewicz) or a smaller radiculomedullary branch16 (Fig. 14-11). Although this vessel typically originates at the T9-T12 level, it may have a more superior take-off from the upper thoracic aorta.17 Very rarely, transverse myelitis with paraplegia may be caused by bronchial artery embolization if this anatomy is present.
Variant anatomy
A variety of congenital anomalies may affect the pulmonary vasculature18–20 (Box 14-3).
Box 14-3 Congenital Anomalies of the Pulmonary Vessels
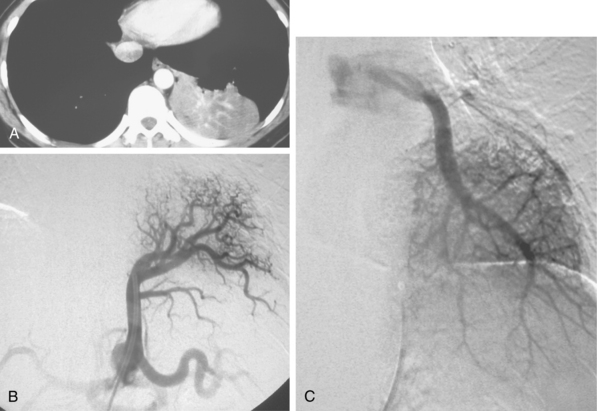
Pulmonary sequestration is an unusual disorder in which a portion of the lung develops independently and derives its blood supply from a systemic artery.21 In intralobar sequestration, the abnormal tissue lies within the visceral pleura of the adjacent lung. The sequestered segment usually is found in the posterior portion of the lower lobe, more commonly on the left side. The systemic blood supply to the sequestration comes from the descending thoracic or abdominal aorta (Fig. 14-12). The venous drainage usually is through the pulmonary veins; inferior vena cava (IVC) and azygous drainage also have been described. In extralobar sequestration, the abnormal segment is contained within its own pleural membrane. The anomaly occurs when splanchnic branches of the aorta that supply the developing lung buds fail to involute. The sequestered segment develops in the left chest in 90% of cases. The systemic arterial supply is from the descending thoracic or abdominal aorta. The sequestration drains into the IVC, azygous veins, or portal venous system. Embolization of the sequestered lung has been performed successfully in neonates.22
Hypogenetic lung (scimitar) syndrome features hypoplasia of the right lung and drainage of blood from all or part of the lung into the IVC (or rarely other systemic veins) at the level of the diaphragm.23 The abnormal lung tissue is fed by branches of the lower thoracic or abdominal aorta. The large draining vein from the mid-right lung to the right cardiophrenic angle produces a C-shaped density on chest radiographs that has been likened to a Turkish sword (scimitar).
Major disorders
Acute pulmonary embolism (online case 8)
Etiology and natural history
Pulmonary embolism (PE) is a common disorder and one of the leading causes of in-hospital mortality.25 PE is part of the spectrum of venous thromboembolic disease (see Chapter 1). Most pulmonary emboli arise from the deep veins of the legs. The remainder come from pelvic veins, the IVC and its tributaries, and upper extremity veins. The last is recognized as an increasingly common source of PE, often following venous thrombosis associated with vascular access devices. Lower extremity venous clots usually originate in the soleal sinuses of the deep veins of the calf. In patients with one or more risk factor for thrombosis (Box 14-4), clot may propagate into the calf, popliteal, and femoral veins (see Chapter 15). The risk for PE with untreated “proximal” (femoropopliteal) deep vein thrombosis approaches 50%.25,26 When embolization occurs, the clot usually shatters while in transit and produces multiple bilateral occlusions of pulmonary artery branches.
Thrombi mechanically impede pulmonary blood flow and lead to the release of a variety of vasospastic activators that intensify the obstruction. The clinical consequences of PE depends on the extent of preexisting cardiopulmonary disease. In patients with previously normal heart and lungs, small emboli produce little effect. However, when the clot burden overwhelms the pulmonary circulation reserve, right ventricular and pulmonary artery pressures rise sharply. This situation may lead to right ventricular dilation, ventricular failure, and ultimately cardiogenic shock. Mortality for patients who present with some hemodynamic compromise is 20% to 30% and approaches 60% in the presence of cardiogenic shock.27 Although this bleak picture usually accompanies massive PE, even small emboli can start such a chain of events in individuals with underlying heart or lung disease. In some patients, inadequate bronchial artery collateral circulation to the obstructed region results in pulmonary infarction.
In patients who survive the acute insult, the natural history of PE is often endogenous lysis of clot over a period of weeks.28 About half of patients experience complete resolution of clot; the remainder have partial resolution or chronic obstruction.29,30 Other nonthrombotic causes of PE include septic foci, fat, air, amniotic fluid, intravascular tumor deposits, and medical devices (e.g., catheter fragments).31–33
Clinical features and diagnosis
PE is a grossly underdiagnosed problem. Physicians must remain alert to the possibility in any patient with cardiopulmonary symptoms or signs and risk factors for venous thromboembolic disease. The most common complaints are chest pain, shortness of breath, cough, tachypnea, and tachycardia. With massive PE, patients may present with syncope, dysrhythmias, or cardiogenic shock. The clinical picture often mimics acute myocardial infarction, pneumonia, exacerbation of chronic obstructive lung disease, or congestive heart failure. Because PE is a common disease and symptoms are nonspecific, screening of patients before requesting radiographic studies is useful. Many experts rely on both clinical probability and laboratory tests to guide decisions about imaging in individuals with suspected PE.25
D-dimer is a breakdown product of cross-linked fibrin. Serum levels are elevated in the setting of vascular thrombosis among many other conditions (advanced age, trauma, pregnancy, operation, inflammation, neolasms).25 The D-dimer test has become a popular screening tool in patients with suspected PE.34,35 The wide range in reported accuracy speaks to differences in assay methods and thresholds for diagnosis. Using higher cutoff (e.g., >500 ng/mL), the negative predictive value of the test approaches 100%.
Imaging
Chest radiography
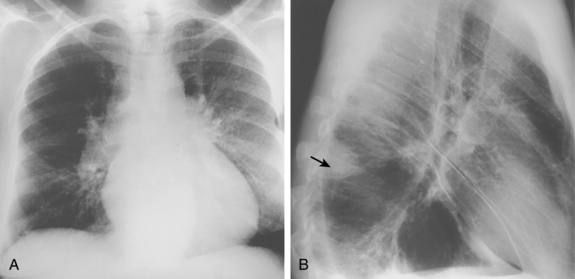
Chest radiographs are useful for suggesting other diagnoses (e.g., pneumonia or congestive heart failure) and are critical for interpretation of radionuclide lung scans. Many patients have nonspecific abnormalities, such as vague parenchymal densities, areas of atelectasis, or pleural effusions. Findings that are more suggestive of PE are rounded or wedge-shaped pleural-based opacities reflecting pulmonary infarction (Hampton hump), local oligemia (Westermark sign), and central pulmonary artery enlargement (Fig. 14-13).
Computed tomography angiography
In most centers, computed tomography (CT) angiography is the principal imaging study for the diagnosis of acute PE.36,37 The sensitivity and specificity with helical single or 4-detector row scanners is 83% to 100% and 92% to 100%, respectively38–44 (Figs. 14-14 and 14-15). Even though the accuracy of commonplace 64-detector row scanners compared with catheter angiography (the gold standard for many decades) has not been rigorously proven, they are considered at least as and probably more accurate for diagnosis than older machines. The negative predictive value of CT angiography approaches 99%.36,45–48 One prospective study (which used clinical probability and D-dimer testing along with imaging) and a recent metaanalysis of the existing literature evaluated the negative predictive value of CT angiography at 3-month follow-up.49,50 The results were remarkably consistent: frequency of missed venous thromboembolic events and fatal PE were 1.3% to 1.4% and 0.5%, respectively. Finally, CT provides a thorough evaluation of the lungs, heart, and mediastinum, which allows diagnosis of many other entities that can mimic PE.
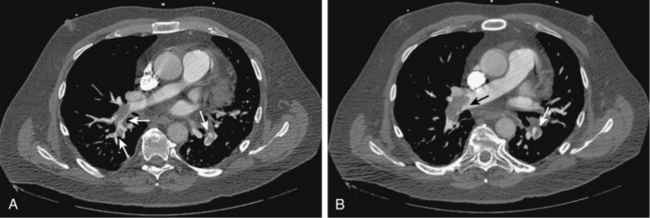
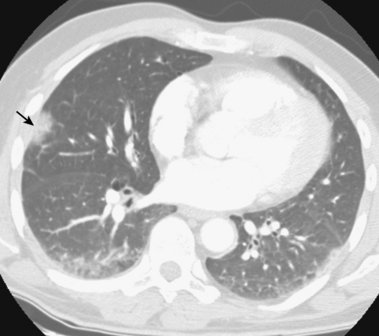
Figure 14-14 A and B, Acute right and left pulmonary artery emboli (arrows) are identified by computed tomography angiography.
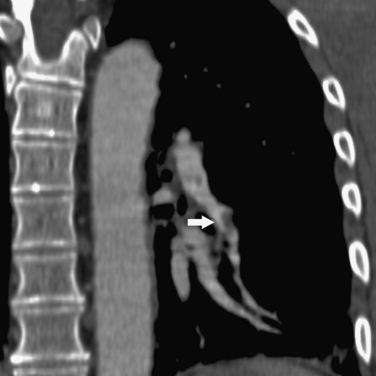
Figure 14-15 Multiplanar reformatted computed tomography angiography displays a segmental pulmonary embolus (arrow).
In some institutions, chest imaging is followed by CT venography of the lower extremities.51,52 Specific scanning and contrast injection protocols are required for CT pulmonary angiography. Lung, mediastinal, and embolism-specific displays must be evaluated.53 Multiplanar reformatted constructions are useful in confirming the diagnosis of clot or the presence of artifacts (see Fig. 14-15). Pulmonary arteries are followed from their origins through the subsegmental branches. Pulmonary artery branches follow their respective bronchi; in the basal segments, medially running pulmonary veins should not be confused with arteries.
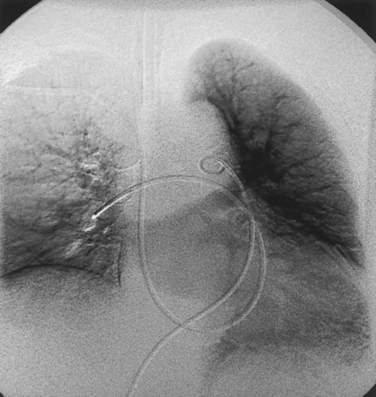
Acute emboli appear as complete, partial, or peripheral filling defects sometimes surrounded by contrast (see Figs. 14-14 and 14-15). The affected vessels are often enlarged. These findings must be distinguished from chronic thromboembolic disease (see later discussion). A peripheral wedge-shaped density may represent an infarct (Fig. 14-16). Signs of right ventricular strain (right ventricular dilation or deviation of the intraventricular septum) should be sought. Table 14-2 outlines potential pitfalls in diagnosis.53
Table 14-2 Misdiagnosis of Pulmonary Embolism on Computed Tomography Angiography
| Cause | Evidence or Response |
|---|---|
| Simulate Bland Acute Pulmonary Embolism | |
| Respiratory motion artifact | “Seagull sign” from vessel motion |
| Image noise | Increase detector width in obese patients |
| Pulmonary artery catheter | Evaluate bone windows |
| Flow-related artifacts | >78 HU |
| Lung algorithm artifact | Inspect standard algorithms |
| Partial volume effect | Evaluate vessels in contiguous images |
| Partial voluming with lymph nodes | Sagittal and coronal reformatted images |
| Lucency in peripheral pulmonary veins | Connection with central pulmonary veins |
| Mucous plug in airway | Note adjacent normal pulmonary artery |
| Perivascular edema | Other signs of heart failure |
| Primary pulmonary sarcoma | Lobulated, enhancing, extravascular spread |
| Tumor emboli (RCC, HCC) | — |
| Chronic pulmonary embolism | (See text) |
| Mask Pulmonary Embolism | |
| Window settings | Window width/level: 700 HU/100 HU |
| Streak artifact (especially near SVC) | — |
HCC, hepatocellular carcinoma; HU, Hounsfield units; RCC, renal cell carcinoma; SVC, superior vena cava.
Radionuclide lung scanning
For many years, ventilation-perfusion (V/Q) lung scanning was widely used for evaluating patients with suspected pulmonary embolus. Because it is less specific than state-of-the-art CT angiography and a definitive diagnosis cannot be made in a substantial number of cases, it has assumed a minor role in most centers. Nonetheless, V/Q scanning can be useful in some situations:54
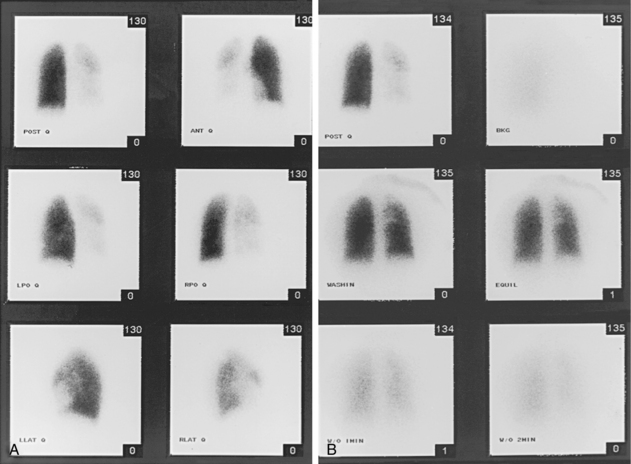
Lung perfusion scans are performed by intravenous injection of technetium-99m (99mTc)–labeled macroaggregated albumin particles. Pulmonary emboli produce localized perfusion defects55 (Fig. 14-17). Perfusion defects also may be seen in areas of lung consolidation, lung collapse, and vasoconstriction because of hypoxia (e.g., chronic obstructive pulmonary disease), among other reasons. Ventilation scanning is done to exclude such entities by imaging after the patient inhales 99mTc-diethylenetriamine-pentaacetic acid aerosol.
Several classification schemes have been proposed for interpreting V/Q scans. A widely accepted one is the system established in the Prospective Investigation of Pulmonary Embolism Diagnosis trials (PIOPED-I and -II), which evaluated the diagnostic usefulness of V/Q scanning for acute PE.56 Two important conclusions can be drawn from the results of the PIOPED-I studies:
Lower extremity duplex sonography
Because most pulmonary emboli arise from the legs and because treatment of PE is aimed at prevention of recurrent embolism, some practitioners initially perform leg duplex sonography to detect potential sources of recurrent emboli. Deep vein thrombosis of the leg is detected in patients with suspected PE less than 25% of the time.57–59 Likewise, up to 50% of patients with proven PE have no sonographic evidence of proximal clot at the time of examination.27 Sonography may be most useful in individuals with equivocal or low probability V/Q scans when clinical signs of deep venous thrombosis or risk factors for venous thromboembolic disease are present.
Magnetic resonance imaging
Promising results have been obtained with MR angiography for PE diagnosis. The reported sensitivity and specificity of gadolinium-enhanced MR angiography is 77% to 100% and 95% to 98%, respectively.60–62 However, multidetector row CT is more accurate than MRI because it has better spatial resolution and produces fewer artifacts.36
Catheter angiography
For decades, pulmonary arteriography was considered the final arbiter in PE diagnosis, but this is no longer true. Multidetector row CT angiography is probably as accurate; in fact, even expert angiographers misdiagnosed isolated subsegmental emboli in up to one third of cases.63–65
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree