Chapter 41 Pulmonary Embolic Disease and Cardiac Tumors
Epidemiology and Risk Factors
PE results from the migration of thrombi formed in the systemic venous system, usually from the pelvis or lower extremities, into the pulmonary arterial system.1,2 These thrombi form as a result of abnormal veins, abnormal blood flow, or abnormalities of the coagulation system. Once formed, fragments of thrombi may become detached from their site of origin as a result of the ongoing coagulation cascade or due to local manipulation. These migrating fragments then become lodged in the pulmonary arterial tree and result in decreased oxygenation capacity in the lungs.
Thrombus formation in abnormal veins may be the result of trauma, iatrogenic manipulation such as catheter access, or venous compression or invasion by tumor.1,2 The presence of a catheter or other foreign body within the vein also predisposes to thrombus formation. Inflammation that involves the vein wall, thrombophlebitis, also produces thrombosis of the vein. The presence of a large thrombus in the vein can also incite an inflammatory reaction in the vein wall and surrounding soft tissues.
Decreased or turbulent flow within veins may instigate thrombus formation. This can be caused by extrinsic compression by a mass lesion or by prolonged positional obstruction of the veins such as in long-distance travel.1,2 In addition, prolonged immobility particularly of the lower extremities results in reduced extrinsic pump function that is normally performed by muscular movements. This results in decreased venous flow, edema, and potential thrombus formation in the affected extremity. Turbulent flow can also contribute to thrombus formation. This can be seen related to foreign bodies such as catheters that may cause thrombosis through chronic inflammation of the vein wall and direct triggering of the coagulation pathways along the surface of the catheter. Central venous catheters are a significant risk factor for thrombus formation with up to 4% of patients with central venous catheters developing catheter-related thrombus.3 The majority of upper extremity DVTs are associated with catheters.4
Abnormalities in the coagulation system are another etiology.1,2 These include various coagulation factor deficiencies or protein abnormalities such as protein C deficiency or Factor V (Leiden factor) abnormalities. Many drugs can alter the coagulation pathway in favor of thrombus formation. This is well known with use of oral contraceptives but also occurs with a variety of chemotherapeutic agents. Examples include methotrexate- and doxorubicin-based regimens as well as thalidomide or its relatives. In addition, hematopoietic stem cell–stimulating agents can also contribute to thrombus formation. Other abnormalities of the hematopoietic system—such as elevated erythrocyte or platelet formation—or abnormalities in the formation of either of these cell lines can also contribute to thrombus formation.
The presence of cancer alone is also associated with a hypercoagulable state. Venous thromboembolism rates are highest with patients on chemotherapy with an incidence as high as 10% in patients with ovarian cancer or lymphoma on therapy and up to 28% in patients with malignant gliomas.3 The highest rates of thrombus formations appear to be in patients with mucinous tumors. However, the frequency of presentation of venous thromboembolism is more closely related to the frequency of the tumor in the population. Most cases of venous thromboembolism in cancer patients are seen in patients with lung, colon, and prostate cancer.
Particular therapies are associated with thrombus formation. Combination therapies including antiangiogenic drugs such as thalidomide are particularly implicated. Thalidomide combination therapy in patients with myeloma has a venous thromboembolism rate approaching 30%, whereas in renal cell cancer, the venous thromboembolism rate is over 40% with this agent.3
Cancer patients are also at greater risk for poor outcomes after PE. For example, they are four to eight times more likely to die after a PE event.3 The 1-year survival for patients with cancer and venous thromboembolism is about one third that of patients with cancer without venous thromboembolism.
Anatomy
DVTs occur most often in the pelvic and lower extremity veins.1,2 Thrombi that form in the upper extremities are more likely to be related to prolonged catheter presence, catheter-related thrombophlebitis, or direct vein involvement by a tumor. Because they are formed within the lumen of vessels, the fragments that are detached are typically tubular in shape. Their size can vary from less than 1 mm in diameter up to 1 or 2 cm in diameter. The larger-diameter thrombi may tend to be longer as well and may have branching components.
Once they are detached, emboli flow through the venous system until they encounter an impeding structure or reach a vessel that is too small to permit their passage. Most commonly, emboli reach and are captured in the pulmonary arterial system. Occasionally, emboli may be captured en route to the pulmonary arterial system, most commonly within the right atrium.5–7 This may occur as emboli become entangled in trabeculations in the right ventricle or right atrial appendage. A congenital Chiari network located at the base of the right atrium between the coronary sinus orifice and the tricuspid valve may entangle a flowing embolus.8 Emboli have also been identified passing through a patent foramen ovale. These “thrombi in transit” can lead to paradoxical emboli to systemic arterial structures including the brain causing embolic strokes.
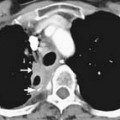
The pulmonary arterial tree is a branching structure with progressively smaller branches. The main pulmonary artery bifurcates into the right and left pulmonary arteries. The right pulmonary artery has three lobar branches and the left has two lobar branches. Each of these branches into two or more segmental branches, and the branching patterns continue out to the periphery of the lung. Emboli that reach the lungs become lodged at arterial branch points or may reach an arteriole of lesser diameter than the embolus (Figure 41-1).
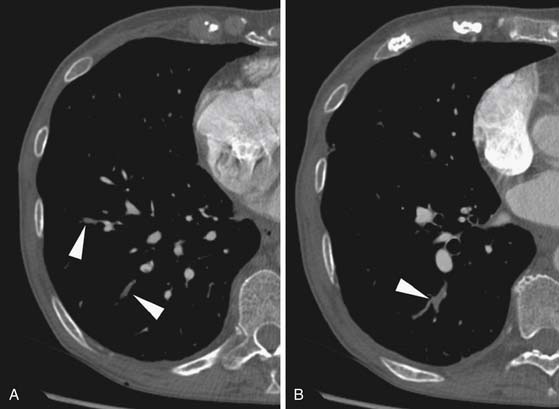
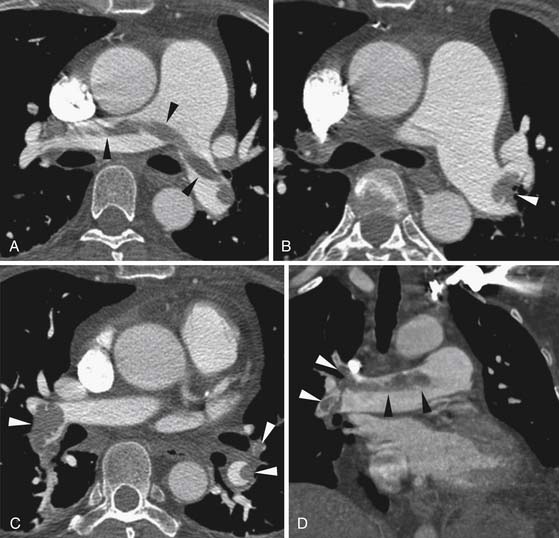
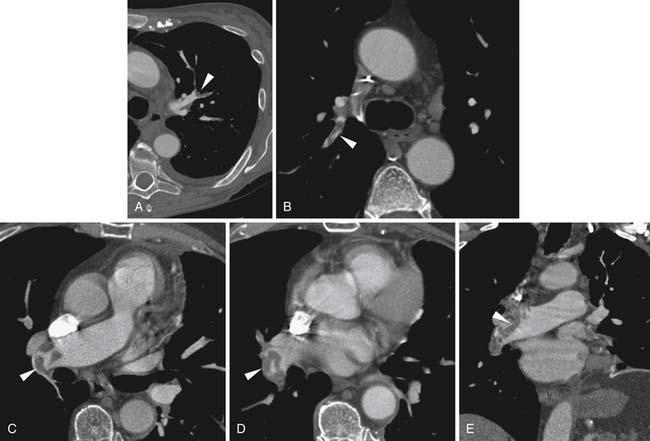
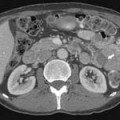
Larger and more elongated emboli will commonly be captured at branch points and may extend into multiple lobes and segments of the pulmonary arterial tree. The largest PEs may become lodged across the main pulmonary artery bifurcation and are called saddle emboli (Figure 41-2). Usually, there is a small bandlike component extending across the pulmonary artery bifurcation with larger components of thrombus extending into the right and left pulmonary arteries and lobar or segmental branches. Smaller emboli may become lodged across branch points at lobar, segmental, or subsegmental levels (Figure 41-3), and still smaller emboli become lodged as they reach subsegmental and smaller arterioles.
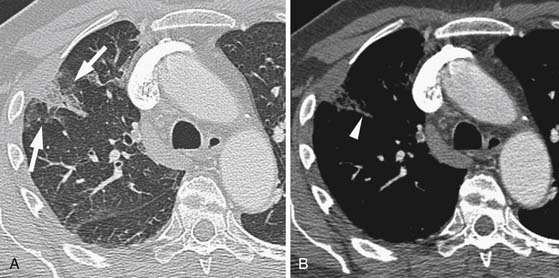
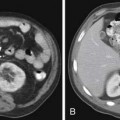
If the embolus completely occludes a segment of the pulmonary arterial system, it can result in infarction of the lung peripheral to that embolus.9 This is relatively uncommon because the lung has a parallel blood supply from the bronchial arteries. Pulmonary ischemia or infarction results in hemorrhage and edema within the affected portion of lung. The involved area typically extends out in a wedge-shaped pattern from the occluded arteriole and reaches the pleural surface (Figure 41-4). The hemorrhagic phase is followed by necrosis and coagulation of the underlying lung architecture. The infarct typically evolves over several days to weeks and may resolve completely or result in a small area of scarring.
Key Points Anatomy
• Emboli are typically elongated “casts” of the vein of origin.
• Emboli tend to become lodged at branch points or become wedged in smaller-caliber arteries.
• Emboli can become entrapped in transit through the right heart chambers or pass through a patent foramen causing systemic embolism.
• Wedge-shaped consolidation peripheral to an occluded vessel represents pulmonary ischemia or infarction and involutes over time.
Clinical Presentation
DVT may be completely asymptomatic. When symptomatic, DVT may present with a variety of nonspecific symptoms including asymmetrical lower extremity swelling and, rarely, an erythematous tender mass along the venous structure.10 Upper extremity thrombosis may also result in asymmetrical edema in the arm, neck, or face.
PEs themselves may be asymptomatic or cause nonspecific symptoms.10,11 The acute onset of dyspnea is the most suggestive symptom and may be accompanied by a sense of impending doom. Pleuritic chest pain also occurs. Larger emboli can result in right heart strain with its symptoms including systemic edema and abnormalities at cardiac auscultation.
Clinical history is helpful in suggesting PE.1,10,11 Any recent period of prolonged immobilization, such as during long travel by automobile or aircraft or after surgery, should suggest the possibility of DVT. A history of cancer or chemotherapy use, particularly those cancers and drugs most associated with thrombosis, also should raise a suspicion of PE in patients with new dyspnea.
Patients with PE often have nonspecific laboratory abnormalities.1,2,10,11 In particular, D-dimer levels are usually elevated. This is a very sensitive but nonspecific finding because cancer patients commonly have abnormalities of D-dimer levels related to their tumor. Coagulation measurements such as prothrombin time, partial thromboplastin time, and the International Normalized Ratio may be abnormal related to the cause of thrombus formation rather than a result of the thrombus or embolus itself. PEs and DVT are more commonly seen in patients with elevated erythrocyte counts and hemoglobin levels as well as in patients with platelet abnormalities of number or function. Arterial blood gas may show an increased difference between alveolar and arterial oxygen concentration called an increased A-a gradient. Pulse oximetry is also often abnormal. Most of these findings are nonspecific and indicate only an abnormality of pulmonary oxygenation, which might be due to pneumonia, embolism, or tumor, among other possibilities.
PEs may be detected on routine imaging of cancer patients without suspicion of PE.12–17 In patients without cancer, PEs are detected in 1% or fewer of the outpatient population and up to 3% of inpatients. Among patients with cancer, approximately 3% to 4% of outpatients and over 6% of inpatients will have unsuspected PE at routine CT scanning. These frequently go undetected because the embolism is not the focus of the examination. Up to 50% to 75% of these will not be seen at clinical interpretation.12,13,16 Even large emboli may be unobserved, particularly in patients with complex presentations of their underlying malignancy. If the PEs are small, subsegmental or smaller, their significance is unknown. Some studies have shown relatively low recurrence rates, particularly compared with the complication rates for therapy, in patients with isolated subsegmental PEs.12,18 These recurrence rates approach that of negative CT pulmonary angiography. They are much less than the recurrence and death rates for patients treated for PE (~8% and 2%, respectively).18 Other studies have shown that anticoagulation for unsuspected PE improves survival.19 Further study is needed to determine the appropriate treatment of isolated subsegmental PEs in particular and unsuspected, asymptomatic PEs in general.
Severity Assessment
Several scoring systems for the extent of pulmonary embolic disease have been proposed. They are generally derived from scoring systems initially proposed for catheter-directed pulmonary angiography.20 The scoring system by Mastora and coworkers21 counts the number of pulmonary arterial segments affected and also modifies the score for the degree of obstruction for each segment. A related scoring system by Qanadli and colleagues22 does not include the degree of obstruction. Both scoring systems correlate with short-term outcomes and the need for intensive care unit admissions.21–26 Assessment of the most proximal level of involvement has also been used as a rating for the severity of embolic disease. The simplified assessment of whether or not the embolism is a saddle embolism has some long-term prognostic value in the assessment of cancer patients.27 Despite the value of these assessments for the extent of PE involvement, the mere presence of emboli is predictive of future embolic events.1 This intermediate- to long-term prognosis is not greatly affected by the severity of the current episode of PE.
The risk of death from an acute episode of PE depends on the hemodynamic state of the patient. Patients with cardiac shock have a high risk of death, normotensive patients with right heart dysfunction have an intermediate risk, and patients without heart dysfunction do not have a high risk.28 Whereas cardiac shock is diagnosed clinically, right heart dysfunction may be evaluated with imaging.
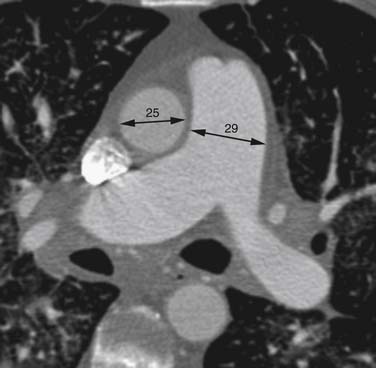
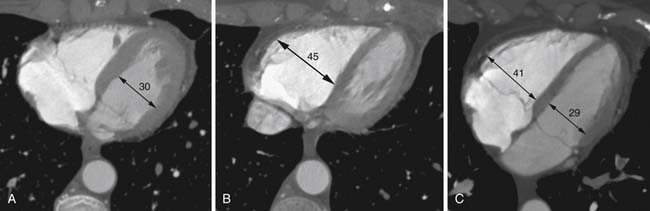
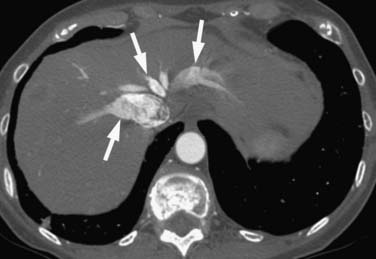
Right heart dysfunction as a result of PE is represented in the development of pulmonary hypertension, right heart strain, and right heart failure. Assessment of these parameters at echocardiography is predictive of survival to hospital discharge and the need for intensive care unit admission.1,2,29 Similar assessments can be done with CT and are also predictive of mortality.23,30–32 This may include simple measurements of the pulmonary artery diameter, which should be less than 33 mm33,34 and less than the aortic diameter (Figure 41-5), and also measurement of the ratio of the right ventricle to left ventricle diameter, which should be less than 1.5 in patients without right heart strain or right heart failure (Figure 41-6).35 However, although pulmonary artery measurements reflect pulmonary hypertension, their value as predictors of PE outcomes had varied.36 If cardiac-gated CT is used, these measurements can be obtained more accurately and additional measurements are also possible.37 Using gated CT, right ventricular ejection fraction compared with left ventricular ejection fraction and pulsatility index of the pulmonary artery can be used to identify decreased right heart function and pulmonary hypertension. However, these measurements have not been thoroughly assessed for their utility in guiding therapy or for their prognostic ability. Secondary signs of right heart strain and failure are less specific but include reflux of contrast material into the inferior vena cava (IVC) and hepatic veins and evidence of systemic edema including hepatic congestion and subcutaneous edema (Figure 41-7).38
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree