Respiratory complications in children infected with human immunodeficiency virus (HIV) are common and responsible for substantial morbidity and mortality. With advances in diagnostic, therapeutic, and preventive strategies for HIV, the spectrum of childhood respiratory disease has changed. In developed countries, programs to prevent perinatal HIV-1 transmission, early diagnosis of HIV-1 infection in infants, and use of Pneumocystis prophylaxis and highly active antiretroviral therapy (HAART) have led to a substantial decline in pediatric HIV incidence and associated respiratory infections. In contrast, the major burden of pediatric HIV now exists in developing countries. In these areas, acute and chronic HIV-associated respiratory disease remain a major cause of childhood morbidity and mortality. This situation is compounded by limited access to appropriate health care and antiretroviral therapy. In the absence of HAART, 90% of HIV-1-infected children may develop a severe respiratory illness sometime in the course of their HIV disease. Pneumonia is the most common cause of hospitalization in African HIV-1-infected children; pneumonia-specific mortality rates are three to six times higher than the rates of HIV-1-negative patients.
The burden of HIV-associated respiratory disease in developing countries often occurs in the context of existing high rates of childhood pneumonia, poverty, coexisting malnutrition, suboptimal immunization coverage, and under-resourced or inaccessible health care facilities. HIV-infected children have a higher risk of respiratory infections and diseases and of more severe illness compared with immunocompetent children. Increasing evidence also suggests, however, that HIV-exposed but uninfected children also are at greater risk of respiratory infections and illnesses compared with children born to uninfected mothers.
Epidemiology
Globally, there are approximately 33.2 million HIV-1-infected individuals, of whom 2.5 million are children younger than 15 years old. Most of these HIV-1-infected children—almost 2.2 million—reside in sub-Saharan Africa. In 2006, there were an estimated 420,000 new HIV-1 infections in children (370,000 in sub-Saharan Africa) and approximately 330,000 childhood deaths from HIV-1 (290,000 in sub-Saharan Africa). Conversely, in the last decade in developed countries, the number of HIV-infected children has greatly declined because of a dramatic reduction in perinatal HIV transmission. New cases of HIV-1 infection in children in developed countries occur predominantly in adolescents secondary to sexual transmission; most adolescents remain asymptomatic until adulthood. HIV-1 infection and AIDS have disproportionately affected minority populations in the United States.
Most new HIV-1 infections in children occur through perinatal transmission in utero , intrapartum, or through breastfeeding. Breastfeeding may account for 40% of infants becoming infected after delivery, especially in developing countries, where mothers continue to breastfeed for prolonged periods or mixed feeding is introduced early.
The impact of HIV-1 on children is compounded by maternal HIV-1 infection. Infection rates in African women are two to five fold higher than in men, with women of childbearing age most affected. In many countries in sub-Saharan Africa, more than 20% of pregnant women are HIV-1-infected. As a result, many HIV-1-exposed children may be cared for by ill mothers, and other caregivers, or be orphaned. Maternal illness and inability to work exacerbates the cycle of poverty and child illness. Children living in sub-Saharan Africa are particularly vulnerable to HIV-1-associated illness because access to antiretroviral therapy and appropriate medical care may be very limited or unaffordable.
Pathogenesis
HIV-1 is a retrovirus, belonging to a group of heterogeneous, lipid-enveloped RNA viruses. Another retrovirus, HIV-2, is relatively rare and causes a less severe AIDS-like syndrome. HIV-1 has two major viral envelope proteins—the external glycoprotein gp120 and the transmembrane glycoprotein gp41.
The primary target cell of HIV-1 is the human CD4 + lymphocyte. The HIV-1 gp120 envelope protein binds to the CD4 + molecule on the host cell membrane with high affinity. This binding allows the virus to enter the T cell and to integrate its genome into the host DNA. HIV-1 also infects monocytes and macrophages but with less marked cytopathic effects. Infected monocytes serve as a reservoir for HIV-1, allowing further spread of the virus throughout the body. Infection with HIV-1 results in progressive depletion of the CD4 + helper lymphocytes. This depletion serves as a marker of the severity of HIV-1 infection because the incidence of opportunistic infections and other complications correlates with the number and percentage of CD4 + lymphocytes, particularly in children older than 1 year.
The ability to produce cytokines, such as interleukin-2 and interferon-γ, is progressively lost in HIV-1-infected children. Natural killer cell–mediated cytotoxicity also is reduced in HIV-1-infected children. In addition, B cell dysfunction with defective humoral immunity further predisposes to severe infection.
Acute Respiratory Infections
Respiratory infection was the most common cause of death in children younger than 6 years of age in a U.S. cohort of HIV-1-infected children in the pre-HAART era; the frequency of pulmonary disease as a cause of death was greatest in infants, with 56% of respiratory-related deaths occurring within the first year of life. The rate of acute respiratory infections and opportunistic infections has decreased dramatically with the use of HAART ( Table 2-1 ). Before HAART, bacterial pneumonia, Pneumocystis pneumonia (PCP), disseminated Mycobacterium avium-intracellulare complex (MAC), and tracheobronchial candidiasis were the most frequent respiratory infections, occurring at an event rate of more than 1 per 100 child-years; these all have declined substantially with HAART (see Table 2-1 ). Although the frequency of bacterial infections has declined substantially, pneumonia or secondary respiratory failure remains the predominant cause of death in children receiving HAART, accounting for 27% of deaths. In children not receiving HAART or children resistant to antiretroviral therapy, acute respiratory infections are common, often severe, and the most frequent cause of hospitalization or death. Bacteria, mycobacteria, viruses, Pneumocystis, or fungi may cause respiratory infections; mixed infections also commonly occur ( Table 2-2 ).
| INFECTION | PRE-HAART RATE | POST-HAART RATE | ||
|---|---|---|---|---|
| Incidence Rate per 100 Child-Years | 95% CI | Incidence Rate per 100 Child-Years | 95% CI | |
| PCP | 1.3 | 1.1‐1.6 | 0.1 | 0.04‐0.2 |
| Bacterial pneumonia | 11.1 | 10.3‐12.0 | 2.2 | 1.8‐2.6 |
| Bacteremia | 3.3 | 2.9‐3.8 | 0.4 | 0.2‐0.5 |
| Disseminated MAC | 1.8 | 1.5‐2.1 | 0.1 | 0.1‐0.3 |
| Tracheobronchial or esophageal candidiasis | 1.2 | 1.0–1.5 | 0.1 | 0.03–0.2 |
| INFECTION | PATHOGENS | FIRST-LINE THERAPY |
|---|---|---|
| Bacterial pneumonia | Streptococcus pneumoniae | Broad-spectrum antibiotic—β-lactam with an aminoglycoside or a second- or third-generation cephalosporin |
| Haemophilus influenzae | Add antistaphylococcal antibiotics if S. aureus suspected or vancomycin if methicillin-resistant S. aureus suspected | |
| Staphylococcus aureus | ||
| Nontyphoid salmonella | ||
| Klebsiella pneumoniae | ||
| Streptococcus milleri | ||
| Escherichia coli | ||
| Moraxella catarrhalis | ||
| PCP | Pneumocystis jiroveci | Trimethoprim-sulfamethoxazole |
| Corticosteroids if hypoxic | ||
| Mycobacterial | Mycobacterium tuberculosis | Isoniazid, rifampin, pyrazinamide as induction for 2 mo (add fourth drug if suspected drug resistance or smear-positive pulmonary TB or smear-negative pulmonary TB with extensive parenchymal involvement or severe extrapulmonary TB or severe concomitant HIV disease); then maintenance with isoniazid, rifampin for 4–7 mo for pulmonary TB |
| Corticosteroids if endobronchial disease, pericardial effusion, meningitis | ||
| Mycobacterium bovis | Surgical excision of localized disease; 4-drug therapy for disseminated disease (isoniazid, rifampin, ethambutol, ofloxacin or ciprofloxacin) | |
| Mycobacterium avium-intracellulare complex | Clarithromycin plus ethambutol | |
| Viral pneumonia | Respiratory syncytial virus | |
| CMV | Ganciclovir for CMV | |
| Human metapneumovirus | ||
| Parainfluenza viruses 1 and 3 | ||
| Adenovirus | ||
| Influenza viruses A and B | Neuraminidase inhibitor for influenza A or B | |
| Measles virus | High-dose vitamin A for measles | |
| HSV | Acyclovir for HSV | |
| Varicella-zoster virus | Acyclovir for varicella-zoster virus | |
| HPV types 6 and 11 | Laser or surgery, topical therapy for HPV | |
| Fungal | Candida species | Topical nystatin or oral itraconazole, fluconazole |
| Aspergillus species | Amphotericin B | |
| Histoplasma capsulatum | Amphotericin B, itraconazole, fluconazole for mild illness | |
| Cryptococcus neoformans | Amphotericin B, itraconazole, fluconazole | |
| Coccidioides immitis | Amphotericin B, itraconazole, fluconazole for mild illness |
Bacterial Pneumonia
Before HAART, bacterial pneumonia was the most common serious infection in HIV-1-infected children with an event rate of 11 per 100 child-years ; this rate has declined to 2.2 in the HAART era (see Table 2-1 ). Pneumonia, particularly caused by Streptococcus pneumoniae, Haemophilus influenzae type b, and Staphylococcus aureus, is a major cause of hospitalization and death in children in developing countries. S. pneumoniae is the most important bacterial pathogen in HIV-1-infected and uninfected children. HIV-1-infected children also are at increased risk for recurrent infections.
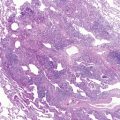
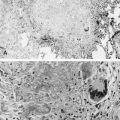
S. aureus is an increasingly important cause of pneumonia in HIV-1-infected children and is the most common pathogen in catheter-associated bacteremia. Staphylococcal pneumonia may be complicated by empyema, pneumatoceles, or lung abscess ( Fig. 2-1 ). A wider range of bacteria, including gram negative pathogens such as Klebsiella pneumoniae, Pseudomonas aeruginosa, H. influenzae, non-typhoid salmonella, and Escherichia coli, cause pneumonia with or without bacteremia in HIV-1-infected children.

HIV-1 infection has been associated with an increase in the antimicrobial resistance patterns of bacterial pneumonia, with implications for empiric antibiotic therapy. Methicillin-resistant S. aureus (MRSA) has increasingly emerged as a pathogen in HIV-1-infected children. Data on the prevalence of penicillin-resistant pneumococcal infection are variable, but no clear differences in clinical outcome for susceptible and resistant strains have been shown except for isolates with high-level resistance.
Etiologic diagnosis of bacterial pneumonia is difficult because signs are nonspecific, coinfection with more than one organism occurs frequently, and culture of upper respiratory tract secretions or sputum may reflect colonization rather than pathogenic organisms. Blood culture, may be useful because of higher rates of bacteremia, occurring in approximately 15% of HIV-1-infected children hospitalized for pneumonia. Empiric therapy for pneumonia should include broad-spectrum antibiotics, based on the local prevalence of antimicrobial resistance and recent use of prophylactic or therapeutic antibiotics (see Table 2-2 ). A combination of a β-lactam with an aminoglycoside or a second-generation or third-generation cephalosporin alone is appropriate empiric therapy. The choice of antimicrobial agent should be modified according to culture results and susceptibility testing.
The outcome of HIV-1-infected children with bacterial pneumonia is worse than the outcome for immunocompetent children with more severe disease and higher case-fatality rates. In addition, HIV-1-exposed but uninfected children have higher rates of treatment failure and mortality compared with children born to uninfected mothers.
Prevention of bacterial pneumonia through immunization is an important strategy, although the efficacy may be reduced in HIV-1-infected children. Immunization with inactivated vaccines (diphtheria, pertussis, tetanus toxoid; inactivated poliovirus; H. influenzae type b; hepatitis B; and pneumococcal conjugate vaccine) should be given to HIV-1-infected children at the usual recommended age. Use of the H. influenzae type b conjugate vaccine potentially may reduce Hib invasive disease by 46% to 93% in immunocompetent vaccine recipients. The efficacy of this vaccine against invasive disease is reduced, however, in HIV-1-infected children not receiving antiretroviral therapy (44% in HIV-infected compared with 96% in uninfected children). The pneumococcal conjugate vaccine has lower efficacy in HIV-1-infected children ; nevertheless, it reduces the incidence of invasive disease caused by vaccine strains by 65% and prevents 13% of radiologically confirmed pneumonia in HIV-1-infected children not receiving HAART. Immunization also reduces the incidence of infection with drug- resistant pneumococcal strains. Reimmunization after 3 to 5 years is recommended. Adolescents should receive the 23-valent polysaccharide vaccine.
Intravenous immunoglobulin (IVIG) is currently recommended in HIV-1-infected children with hypogammaglobulinemia (IgG <4 g/L); two or more severe bacterial infections in a 1-year period; failure to form antibodies to common antigens; or chronic parvovirus B19 infections. IVIG may not offer additional protection, however, if children are taking trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis. Moreover there is no evidence to suggest that IVIG therapy offers additional protection for children receiving HAART. Children with bronchiectasis may benefit from monthly immunoglobulin infusions.
Mycobacterial Infection
Respiratory disease also may result from infection with numerous mycobacteria. Mycobacterium tuberculosis is the most common cause of respiratory infection, but localized or disseminated disease from Mycobacterium bovis or the nontuberculous mycobacteria (NTM), particularly MAC may occur (see Table 2-2 ).
In high tuberculosis (TB) prevalence areas, M. tuberculosis is an important cause of acute pneumonia and of chronic respiratory infection in HIV-1-infected children. Culture-confirmed pulmonary TB has been reported in approximately 8% to 15% of children hospitalized with acute pneumonia in these areas. The incidence of TB usually primary infection, than in non-HIV infected children is higher in HIV-1-infected children. Coinfection with M. tuberculosis and HIV-1 results in more rapid deterioration of immune dysfunction, viral replication, and HIV-1 progression, and other more frequent and severe infections.
HIV-1-infected children with TB may present with nonspecific signs, such as weight loss, failure to thrive, or fever; with signs and symptoms of acute pneumonia or airway obstruction ( Fig. 2-2 ); or with chronic, persistent respiratory symptoms. Cavitary lung disease, or extrapulmonary spread. ( Fig. 2-3 ) occurs more commonly in HIV-1-infected children. Multidrug-resistant TB is increasingly prevalent in TB-endemic areas and has a poor prognosis. In the United States, multidrug-resistant TB is uncommon, reported in 2.8% of foreign-born and 1.4% of U.S.-born cases.



Localized or disseminated M. bovis infection, including pneumonia, has been reported in HIV-1-infected children occurring weeks to years after receiving bacille Calmette-Guérin (BCG) immunization. The most common manifestation of M. bovis disease is ulceration at the site of vaccination or localized lymphadenopathy; systemic dissemination occurs more rarely. The risk of disseminated BCG disease is greatly increased in HIV-1-infected infants. The clinical presentation of disseminated M. bovis may be indistinguishable from M. tuberculosis infection. Disseminated M. bovis infection has a poor prognosis with a case-fatality rate of approximately 50%.
NTM, particularly MAC, may cause disseminated disease, including pulmonary infection, in severely immunosuppressed HIV-1-infected children; isolated pulmonary disease is rare. The incidence of NTM disease has declined significantly from a rate of 1.8 per 100 child-years before HAART to 0.1 after HAART (see Table 2-1 ). Children with pulmonary disease are at high risk for dissemination; 72% may develop systemic disease within 8 months. Disseminated MAC seems to be more common in children who have transfusion-acquired HIV-1 rather than perinatal acquisition. Disease occurs in adults with CD4 + counts less than 50 cells/μL; however, the threshold is less well established in young children. Primary and secondary prophylaxis is recommended for severely immunosuppressed children based on CD4 + counts.
Establishing the diagnosis of pulmonary TB is difficult in HIV-1-infected children, for whom clinical scoring systems have not been developed, and in whom anergy may reduce the reliability of the tuberculin skin test. A tuberculin skin test of 5mm or more of induration is regarded as positive. Tests of T lymphocyte interferon-γ production are promising. A study of children with suspected TB reported that the T cell–based enzyme-linked immunospot assay (ELISPOT) had a higher sensitivity than the tuberculin skin test. In HIV-1-infected children, the ELISPOT sensitivity was 73% compared with 36% for the skin test. Definitive diagnosis and drug resistance testing require culture confirmation of M. tuberculosis . Sputum induction was reported more recently to be effective and safe for culture confirmation in infants and children, with the yield from a single induced sputum equivalent to that obtained from three gastric lavages. Induced sputum should be the primary diagnostic procedure in a child with suspected pulmonary TB. In contrast, the culture yield from a single bronchoalveolar lavage (BAL) is lower than that from three properly performed consecutive gastric lavages. The efficacy of polymerase chain reaction has been disappointing, with sensitivity on gastric aspirates ranging from 45% to 83% in HIV-1-negative children.
Definitive diagnosis of M. bovis or MAC relies on isolation of the organism from the blood or biopsy specimens. If lymphadenopathy is present, an aspirate and culture can be diagnostic. Multiple mycobacterial blood cultures may be necessary to improve the yield. Culture is essential to differentiate NTM from M. tuberculosis and to determine drug susceptibilities.
The response to standard TB therapy in HIV-1-infected children is poorer than in HIV-1-negative children, with lower cure rates and higher mortality. Optimal therapy for HIV-1-infected children has not been tested in well-designed clinical studies. Empiric therapy for pulmonary TB in HIV-1-infected children should include three drugs (isoniazid, rifampin, pyrazinamide) daily for a 2-month induction period; a fourth drug (ethambutol, ethionamide, or streptomycin) should be added if drug resistance is suspected or if there is smear-positive pulmonary TB, smear-negative pulmonary TB with extensive parenchymal involvement, severe extrapulmonary TB, or severe concomitant HIV-1 disease.
After the induction phase, therapy with two drugs (isoniazid, rifampin) should be continued either daily or three times a week. Directly observed therapy (DOT) is advised to promote adherence and reduce the rate of treatment relapse or failure. Because high rates of treatment failure occur in children treated for 6 months, some guidelines recommend 9 months. For extrapulmonary TB, the duration of therapy may be increased to 12 months. Therapy for drug-resistant TB should be individualized, using a minimum of three drugs, at least two of which are bactericidal. Adjunctive corticosteroids may be beneficial in children with complicated TB, including pericardial disease, meningitis, or an endobronchial lesion with airway obstruction; a suggested regimen is 1 to 2mg/kg/day of prednisone tapered over 6 to 8 weeks. A chest radiograph should be obtained at baseline and repeated 2 to 3 months into therapy to evaluate response; however, the chest radiograph may remain abnormal for months even years, and a normal radiograph is not a criterion for discontinuing therapy.
For children receiving HAART, the antiretroviral regimen should provide optimal TB and HIV-1 therapy and minimize potential toxicity and drug interactions. Rifampin induces hepatic cytochrome P-450 enzymes and may reduce levels of antiretroviral agents, particularly protease inhibitors (Pls) and non-nucleoside reverse transcriptase inhibitors (NNRTIs). Rifampin should not be used in conjunction with single protease inhibitors except for ritonavir. Alternatively, rifampin may be used in conjunction with ritonavir-boosted saquinavir, provided that high-dose ritonavir boosting (400mg) is used. Concurrent rifampin with the non-nucleoside reverse transcriptase inhibitor delavirdine is not recommended; however, use with efavirenz is possible. Use with nevirapine is recommended only when there are no other options because of the potential decrease in nevirapine levels. Rifabutin is a less potent inducer of the P-450 enzymes, and is a suitable alternative to rifampin, but there is limited experience with its use in children. Adjustments in dosage of rifabutin and coadministered antiretroviral drugs may be necessary because some drugs decrease rifabutin levels, whereas others increase levels. For antiretroviral-naïve children, the timing of HAART after initiation of TB therapy depends on the clinical and immunologic severity of disease. In children with severe clinical illness or advanced HIV-1 infection, HAART should be started 2 to 8 weeks after TB therapy to minimize the risk of immune reconstitution inflammatory syndrome (IRIS), to optimize adherence, and to differentiate potential side effects secondary to TB or antiretroviral drugs.
Management of BCG disease is difficult. The inherent resistance of M. bovis to pyrazinamide, inherent intermediate resistance of some strains to isoniazid, and the emergence of resistance owing to inappropriate therapy complicate treatment. Although localized BCG disease is usually self-limited in immunocompetent children, in HIV-1-infected children, treatment is warranted because of the risk of dissemination and poor outcome. Surgical excision of localized lymphadenopathy is one option. Alternatively, therapy with four drugs (isoniazid, rifampin, ethambutol, ofloxacin, or ciprofloxacin) in high doses is recommended. The optimal duration of therapy is unknown, but at least 9 months of treatment is recommended, based on experience with adults.
Treatment of MAC should consist of combination therapy with a minimum of two drugs because monotherapy with a macrolide leads rapidly to resistance. Initial recommended therapy is clarithromycin or azithromycin with ethambutol. Rifabutin may be added as a third drug in patients with severe disseminated infection; addition to ciprofloxacin, amikacin, or streptomycin may be considered depending on the severity of illness.
For prevention of mycobacterial disease, the BCG vaccine may be beneficial for HIV-1-exposed but uninfected children in areas of high TB prevalence. Chemoprophylaxis with isoniazid is recommended for a child who has been exposed to a household contact with TB after active TB disease has been excluded or for a child with evidence of TB infection (tuberculin skin test >5mm induration but asymptomatic with a normal chest radiograph). A study in a high TB prevalence area found that isoniazid prophylaxis given to HIV-1-infected children regardless of tuberculin skin reactivity or a household TB contact substantially reduced TB incidence by almost 70% and mortality by more than 50%. The effect of isoniazid occurred in all categories of clinical HIV-1 illness and in children of all ages. Isoniazid prophylaxis should be considered for HIV-1-infected children living in TB endemic areas, especially children who are not taking HAART.
Primary prophylaxis for NTM with azithromycin or clarithromycin should be considered for severely immunosuppressed children ( Table 2-3 ) as follows: children younger than 1 year, CD4 + less than 750/μL; children 1 to 2 years old, CD4 + less than 500/μL; children 2 to 6 years old, CD4 + less than 75/μL; children 6 years or older, CD4 + less than 50/μL. Rifabutin may be an alternative agent in children older than 6 years. Secondary prophylaxis should be given to children with a history of disseminated MAC to prevent recurrence. Lifelong prophylaxis is indicated; the safety of discontinuing secondary prophylaxis in the context of sustained immune restoration after HAART has not been well studied in children.
| AGE | CD4 + T LYMPHOCYTE COUNT * |
|---|---|
| 4–6 wk to 12 mo † | All patients regardless of CD4 + count |
| 1–5 yr | <500/mm 3 or <15% |
| >5 yr | <200/mm 3 or <15% |
* If CD4 + counts are unavailable, prophylaxis should be given to all symptomatic children indefinitely.
† HIV-exposed children should receive prophylaxis from 4–6 weeks to 4 months; thereafter, prophylaxis may be discontinued if HIV infection has been excluded, and the mother is not breastfeeding.
Viral Infection
Although respiratory viruses are identified less frequently in HIV-1-infected children hospitalized for pneumonia (approximately 15%) compared with HIV-1-negative children (45%), the absolute burden of hospitalization for viral-associated pneumonia is twofold to eightfold greater in HIV-1-infected children. HIV-1-infected children in whom respiratory viruses are identified are more likely to develop pneumonia, to have a more prolonged hospital stay, and to have a higher case-fatality rate than HIV-1-uninfected children. Respiratory syncytial virus (RSV) is the most common cause of viral pneumonia, although human metapneumovirus is emerging as an important respiratory pathogen with a spectrum of disease similar to RSV. Other respiratory viruses causing lower respiratory tract infection include cytomegalovirus (CMV), adenovirus, influenza, parainfluenza, and measles (see Table 2-2 ). Treatment may be beneficial for specific viral pathogens. For influenza A or B, a neuraminidase inhibitor, such as oseltamivir or zanamivir, can reduce the severity of illness and complications.
Concurrent bacterial infection has been reported in 30% to 50% of children hospitalized with viral pneumonia. Pneumococcal conjugate vaccine reduces the incidence of hospitalization for viral-associated pneumonia, suggesting that more severe pneumonia requiring hospitalization may be the result of viral and S. pneumoniae coinfection. Influenza vaccine should be given annually to all HIV-1-infected children at the start of the influenza season. The efficacy of the humanized monoclonal specific antibody against RSV (palivizumab) or RSV IVIG has not been well evaluated in HIV-1-infected children. Nevertheless, children at risk for severe RSV infection, such as HIV-1-infected infants born prematurely, children younger than 2 years with chronic lung disease, or severely immunosuppressed children, may benefit from palivizumab prophylaxis. A dose should be given monthly for the duration of the RSV season.
CMV is a herpesvirus that can cause primary pneumonitis or severe, disseminated disease in HIV-1-infected children. CMV infection is more likely to occur in children with low CD4 + counts. Coinfection with CMV and HIV-1 results in more rapid progression of HIV-1 disease. The incidence of CMV infection has decreased with the use of HAART. CMV may occur in association with other pathogens, especially Pneumocystis . Treatment of CMV disease focuses on preventing disease progression and not on cure. Ganciclovir is most widely used, with drug dosing separated into induction and maintenance dosage. Prophylaxis against CMV with oral ganciclovir or valganciclovir should be given to severely immunosuppressed children or children with a history of disseminated CMV disease to prevent recurrence. There are few data on the safety of discontinuing prophylaxis after sustained immune reconstitution on HAART has occurred.
Other herpesvirus infections may also involve the respiratory tract in HIV-1-infected children. Oral herpes simplex virus lesions may spread to involve the upper airways and the larynx, resulting in croup ; disseminated disease including pneumonia also may occur. Pneumonia may occur as a complication of varicella-zoster virus infection. Intravenous acyclovir is recommended for therapy; high-dose oral acyclovir or valacyclovir may be considered in children with mild immunosuppression. Varicella vaccine should be considered at 12 to 15 months for asymptomatic or mildly symptomatic HIV-1-infected children without immunosuppression (Centers for Disease Control and Prevention categories N1 and A1); vaccine should not be administered to symptomatic immunosuppressed children because of the potential for disseminated disease. Administration of varicella-zoster globulin should be considered for HIV-1-infected children exposed to varicella or zoster who have no prior history of varicella infection or immunization and who have not received immunoglobulin within 2 weeks of exposure.
Measles may result in severe pneumonia in HIV-1-infected children; measles may present without the typical skin rash, making the diagnosis particularly difficult. Children with suspected measles should be given a single high dose of vitamin A because studies in HIV-1-negative children have shown that vitamin A reduces morbidity and mortality from measles-associated pneumonia. Measles, mumps, rubella vaccine (MMR), a live attenuated vaccine, should be given to HIV-1-infected children at 12 months of age, unless they are severely immunocompromised. HIV-1-infected children exposed to measles should receive a dose of intramuscular immunoglobulin regardless of immunization status.
Human papillomavirus (HPV) type 6 or type 11 may produce lesions in the oral cavity, pharynx, and larynx and rarely in the lower airways or lungs; the disease has a tendency to recur. Clinically, the disease may manifest as progressive hoarseness, stridor, wheezing, and respiratory distress. Rarely, lung nodules, cysts, recurrent pneumonia, emphysema, or atelectasis occurs in immunocompetent children. Little is known about the epidemiologic risk of disease in HIV-1-infected children. An increased prevalence of HPV in HIV-1-infected compared with HIV-1-uninfected women has been reported; however, the rate of HPV transmission to children has not been associated with the HIV-1 status of the mother or child. Laryngeal HPV lesions are difficult to treat. Therapy is directed at maintaining airway patency, so obstructing papillomas should be removed. Adjuvant therapy using intralesional cidofovir has been reported to result in regression and reduced need for surgery in HIV-1-uninfected children.
Pneumocystis Infection
Children with HIV-1 who are not taking HAART or TMP-SMX prophylaxis are at high risk of developing PCP. Pneumocystis jiroveci (formerly Pneumocystis carinii ) originally was classified as a protozoan. Studies of RNA sequences show a similarity between fungi and P. jiroveci, and the organism is now classified as a fungus. Two morphologic forms of the organism are found in infected lungs: thin-walled, single-nucleated trophozoites adherent to type I pneumocytes, and thick-walled cysts containing four to eight single-nucleated sporozoites. The organism attaches to the alveolar epithelium, resulting in desquamation of alveolar cells. As the infection progresses, a diffuse desquamative alveolitis ensues, and alveoli become filled with a foamy exudate consisting of alveolar macrophages and cysts. Interstitial inflammation develops.
PCP was the most common opportunistic infection in HIV-1-infected infants before widespread prenatal HIV-1 screening, TMP-SMX prophylaxis, and HAART. In the United States, the incidence of PCP has declined substantially from 1.3 per 100 child-years before HAART to 0.1 after HAART. In developed countries, PCP occurs most commonly in infants born to women with unrecognized HIV-1 infection. In developing countries, PCP remains a frequent presentation of HIV-1 infection in infants and a major cause of severe pneumonia and death. The incidence of PCP ranges from 8% to 49% among HIV-1-infected African children hospitalized for pneumonia. Increasingly, PCP also has been reported in older HIV-1-infected children; 25% of cases in a Zambian postmortem study occurred in children older than 6 months. Moreover, HIV-1-exposed but uninfected children have been described to have a higher risk of PCP compared with children born to HIV-1-uninfected mothers.
Children with PCP most commonly have acute onset of cough, fever, tachypnea, and chest retractions. Infants younger than 6 months are especially at risk for PCP and have an acute, severe illness characterized by rapidly prominent and progressive hypoxia and increasing respiratory distress. In HIV-1-infected children not taking HAART, four clinical variables have been reported to be associated with PCP: age younger than 6 months, respiratory rate greater than 59 breaths/min, arterial hemoglobin saturation less than 92%, and absence of a history of vomiting. Clinical signs may be compounded by coinfection with bacterial or viral pathogens. Most children have significant hypoxemia with an alveolar-arterial oxygen gradient greater than 30mmHg.
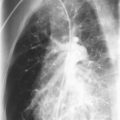
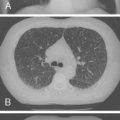
Laboratory findings include a normal white blood cell count, elevated serum lactate dehydrogenase (LDH), and normal IgG level. LDH values greater than 1000 IU/L are associated with PCP, but they are nonspecific and may reflect the extent of lung involvement. The chest radiograph usually shows a diffuse pattern, which progresses to alveolar opacification ( Fig. 2-4 ); however, hyperinflation, focal infiltrates, cavities, a miliary pattern, pneumothoraces, pleural effusion, or even a normal appearance also may occur. Less commonly, PCP may manifest with a pneumothorax, cyst, pneumatoceles, or a bronchiolitis-like picture.

PCP is associated with high mortality ranging from 35% to 87% with higher rates in children with respiratory failure requiring mechanical ventilation. Timely anti- Pneumocystis therapy may improve the outcome, as suggested by historical comparisons and adult studies in which early use of corticosteroids has been associated with improved survival. Mutations in P. jiroveci dihydropteroate synthetase genes—a key enzyme target of TMP-SMX—have been described in HIV-1-infected patients with PCP, especially with widespread use of TMP-SMX prophylaxis. The clinical importance of mutant strains is unclear, however, and the response to TMP-SMX treatment varies.
HIV-1-exposed but uninfected children may also be at increased risk of PCP. Transmission of P. jiroveci from an HIV-1-infected mother to her HIV-1-uninfected infant has been reported in a few cases. HIV-1-exposed children may be at risk for PCP as a result of close and early exposure to the organism from the mother, reduced passage of functional maternal antibody, impaired cell-mediated immunity, or concomitant malnutrition.
Definitive diagnosis requires identification of P. jiroveci from sputum, bronchial washings, or lung tissue. Induced sputum using nebulized hypertonic saline has been used to diagnose PCP in children; a positive yield has been reported in infants 1 month of age. In this procedure, the child inhales a mist of 3% to 5% saline generated by a jet nebulizer for 10 to 15 minutes. The diagnostic yield using this technique depends on collection of an adequate specimen. Nasopharyngeal aspirates have been used to identify P. jiroveci, but the yield is lower than with induced sputum. Induced sputum combined with nasopharyngeal aspirates may provide a higher yield than either technique alone; the sensitivity and specificity for induced sputum and nasopharyngeal aspirates for diagnosis of PCP compared with the yield on autopsy have been reported to be 75% and 80%. BAL with fiberoptic bronchoscopy is the diagnostic procedure of choice in young children, with reported sensitivity of 55% to 97%. Transbronchial biopsy is not recommended unless BAL is nondiagnostic. Transbronchial biopsy may be positive 10 days after starting therapy; the sensitivity of biopsy is 87% to 95%.
Because P. jiroveci cannot be cultured, identification requires special stains. Silver methenamine, toluidine blue O, and calcofluor white are useful for staining cyst forms, whereas Giemsa, modified Wright-Giemsa, and modified Papanicolaou stains identify trophozoites. Fluorescein-conjugated monoclonal antibodies provide greater sensitivity, detecting the cyst and trophozoite forms. Polymerase chain reaction techniques with a high sensitivity and specificity and potential to improve diagnostic accuracy are promising, but currently are used mainly as a research tool.
Empiric therapy for Pneumocystis should be given to any HIV-1-infected child with suspected PCP because untreated infection is usually fatal. The most effective therapy is TMP-SMX (15 to 20mg/kg/day of TMP) intravenously three to four times a day for 21 days (see Table 2-4 ). Oral treatment can be used if intravenous therapy is not feasible, if disease is mild, or after clinical improvement occurs. The response to therapy may be slow with clinical improvement observed by 5 to 7 days. Adverse reactions to TMP-SMX occur in approximately 15% of cases, but treatment should be discontinued only if reactions are severe, such as neutropenia or a severe skin rash.
| DRUG | DOSE | ROUTE | COMMENTS |
|---|---|---|---|
| TMP-SMX | 15–20mg/kg TMP/75–100mg/kg SMX per day q6h | Intravenous o r oral | First choice. Oral therapy only if mild disease or after clinical improvement occurs |
| Pentamidine | 4 mg/kg once daily | Intravenous | Use in children who cannot tolerate TMP-SMX or when no response after 5–7 days. High incidence of side effects. Should not be administered with didanosine owing to risk of pancreatitis |
| Atovaquone | 30–45 mg/kg/day | Oral | Limited experience in children |
| Trimetrexate glucuronate with leucovorin | No studies in children of established doses. Adult dose 45mg/m 2 /day trimetrexate glucuronate with 20mg/m 2 leucovorin q6h | Intravenous | Limited experience in children |
| Dapsone and trimethoprim | No studies in children of established doses. Adult dose 100mg dapsone daily (pediatric equivalent 2mg/kg) and 15mg/kg trimethoprim | Oral | Limited experience in children |
| Primaquine and clindamycin | No studies in children of established doses. Primaquine adult dose 30mg daily orally (pediatric equivalent 0.3mg/kg daily orally). Clindamycin adult dose 600mg intravenously q6h for 10 days, then 300–450mg orally q6h for 11 days (pediatric equivalent 10mg/kg q6h orally or intravenously) | Oral/intravenous | Limited experience in children. Most effective alternative therapy for adults with PCP unresponsive to primary therapy |
* Corticosteroids should be added for children with hypoxia.
Intravenous pentamidine (4mg/kg/once daily) may be an alternative treatment for children who cannot tolerate TMP-SMX, or who have not responded after 5 to 7 days of TMP-SMX (see Table 2-4 ). Pentamidine is associated with a high incidence of adverse reactions, including pancreatitis, hyperglycemia and hypoglycemia, renal dysfunction, cardiac dysrhythmias, fever, neutropenia, and hypotension. Patients who show clinical improvement after 7 to 10 days of intravenous pentamidine may be switched to an oral drug to complete 21 days of therapy. Other alternative anti- Pneumocystis agents include atovaquone, dapsone with trimethoprim, trimetrexate glucuronate with leucovorin, and clindamycin with primaquine, but there is little information on the efficacy or tolerability of these regimens in children (see Table 2-4 ).
Corticosteroids are recommended in hypoxemic children. Although no controlled trials on the use of corticosteroids in children have been performed, corticosteroid use has been reported to reduce the need for mechanical ventilation and to improve survival compared with historical controls. Studies of hypoxemic HIV-1-infected adults with PCP found that corticosteroids improve oxygenation and reduce the incidence of respiratory failure when used within 72 hours of starting anti- Pneumocystis therapy. Corticosteroids are recommended for patients with a PaO 2 less than 70mmHg or an alveolar-arterial oxygen gradient greater than 35mmHg. The optimal dose and duration have not been determined, but a recommended regimen is prednisone, 2mg/kg/day for 7 to 10 days with tapering doses over the next 10 to 14 days.
A few case reports have described the use of surfactant to improve pulmonary function in children with severe PCP. Children with PCP may be co-infected with bacterial or viral pathogens ; additional antimicrobial therapy for these should be used when appropriate. Specifically, CMV co-infection has been associated with more severe disease requiring mechanical ventilation and a poor outcome. The effect of corticosteroid therapy for PCP on CMV pneumonitis is unclear.
Because of the high mortality and morbidity associated with PCP, prevention should be the primary objective. Prevention of PCP is an important and effective intervention, if initiated in HIV-1-exposed infants within the first weeks of life. Oral TMP-SMX is the most effective prophylactic agent. In the only randomized controlled study of TMP-SMX prophylaxis in HIV-1-infected children, mortality was reduced by 43% and morbidity, including hospitalization, was reduced by 23% in Zambia. The impact on mortality occurred in children of all ages, suggesting that prophylaxis may also provide protection against bacterial infections. TMP-SMX prophylaxis (150mg/m /day of TMP) may be given three times a week (single dose on 3 consecutive days, or two divided doses on consecutive or alternate days).
Current recommendations for PCP prophylaxis include the following (see Table 2-3 )
- •
All infants born to HIV-1-infected mothers from 6 weeks of age until HIV-1 infection has been excluded in the child and the mother is no longer breastfeeding.
- •
All HIV-1-infected children from 6 weeks of age until 1 year. HIV-1-infected children older than 1 year should receive prophylaxis if their CD4 + counts are less than 15% of lymphocytes or if they have symptomatic HIV-1 disease. A higher CD4 + threshold for providing prophylaxis may be applicable in developing countries, however, as evidenced by a trial in Zambia where prophylaxis reduced mortality in children, even in children with higher CD4 + counts. Prophylaxis should be continued indefinitely regardless of age or CD4 + counts when HAART is unavailable.
- •
Prophylaxis should be continued in children taking HAART for at least 6 months. There is little information on the safety of discontinuing prophylaxis after immune reconstitution has occurred. Discontinuation of prophylaxis may be considered in children with confirmed immune restoration for 6 months or more as indicated by two measurements of CD4 + greater than 25% at least 3 to 6 months apart in children 2 to 6 years old.
Lifelong prophylaxis should be given to all children who have had an episode of PCP; the safety of discontinuing secondary prophylaxis in the context of immune reconstitution has not been established. If TMP-SMX is not tolerated or cannot be used, alternatives include dapsone (2mg/kg once daily), parenteral pentamidine (4mg/kg every 2 to 4 weeks), or aerosolized pentamidine (300mg via Respigard II inhaler every 4 weeks) if the child is older than 5 years. Safety and efficacy concerns regarding aerosolized pentamidine preclude its use in younger children.
Fungal Infection
Chronic Candida infection is common in HIV-1-infected children and may produce oropharyngeal, laryngeal, or esophageal candidiasis and promote the development of gastroesophageal reflux disease. The incidence of tracheobronchial or esophageal candidiasis has declined substantially with HAART (see Table 2-1 ). Infection of the upper airways may result in Candida supraglottitis, epiglottitis, and a croup-like picture. Laryngeal candidiasis may manifest as severe acute airway obstruction. Pulmonary disease may also occur in the context of severe disseminated disease. Uncomplicated oropharyngeal candidiasis can be treated with topical therapy (see Table 2-2 ). Oral fluconazole, itraconazole, or ketoconazole are effective alternative agents. For esophageal disease, fluconazole or itraconazole is recommended.
Other fungal infections, including aspergillosis, histoplasmosis, cryptococcosis, and coccidioidomycosis, may produce respiratory illness usually in the context of severe immunosuppression and disseminated disease (see Table 2-2 ). Pulmonary cryptococcosis without dissemination may manifest with fever, intrathoracic adenopathy, and pulmonary infiltrates. Occasionally, pulmonary cryptococcosis may be asymptomatic and manifest on routine chest x-rays as pulmonary nodules. Pulmonary coccidioidomycosis may produce nodules, cavities, or diffuse reticulonodular infiltrates associated with fungemia and systemic disease. Children with severe pulmonary cryptococcosis should be treated with amphotericin B; maintenance therapy with fluconazole or itraconazole can be substituted after improvement has occurred. Mild or moderate pulmonary cryptococcosis can be treated with oral fluconazole or itraconazole. Lifelong suppressive therapy with fluconazole or itraconazole is necessary to prevent relapse. There are few data on treatment of pulmonary coccidioidomycosis in children, and recommendations are based on adult data, with amphotericin B recommended for the acute illness followed by long-term suppressive therapy with fluconazole or itraconazole. Alternatively, in mild disease therapy may be initiated with fluconazole or itraconazole.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree