Respiratory diseases are important causes of morbidity and mortality among children throughout the world. Although diseases attributed to parasitic infection are uncommon for many physicians in temperate climates, these infections remain important conditions that must be addressed. Because of the influx of immigrants from developing countries and the volume of global travel, including children, physicians should have a basic understanding of respiratory illnesses attributed to parasitic infections.
The spectrum of syndromes related to parasitic respiratory infection is quite large. Diagnosis of a parasitic infection should be classified through the recognition of identifiable host characteristics, underlying medical conditions, and specific clinical manifestations. The etiology of acute respiratory infections caused by parasitic agents can be suggested, however, through knowledge of the specific geographic locale of the ill child or the location from which the child has arrived ( Table 13-1 ). Clinical presentations, findings on imaging studies, or individual laboratory results can differentiate infection among particular parasitic organisms. Underlying immunodeficiency should suggest infection with certain organisms, such as Strongyloides stercoralis or Toxoplasma gondii. In patients from appropriate geographic locations with respiratory symptoms and elevated peripheral eosinophil counts, infection with Ascaris lumbricoides, hookworm, S. stercoralis, or Toxocara species should be considered. This chapter reviews parasitic diseases that have pulmonary manifestations.
| GEOGRAPHIC DISTRIBUTION | ||||||
|---|---|---|---|---|---|---|
| ORGANISM | AF | AM | AS | E | OC | WS |
| Helminthic | ||||||
| Ascariasis | X | |||||
| Capillariasis hepatica | X | X | X | X | ||
| Dirofilariasis | X | |||||
| Echinococcosis | X | X | X | X | X | |
| Filariasis | X | X | X | |||
| Gnathostomiasis | X * | |||||
| Hookworm | X | |||||
| Paragonimiasis | X | X | X | X | X | |
| Schistosomiasis † | X | X | X | |||
| Strongyloidiasis | X | |||||
| Toxocariasis | X | |||||
| Trichinellosis ( Trichinella spiralis ) | X | |||||
| Protozoan | ||||||
| Amebiasis | X | |||||
| Cryptosporidiosis | X | |||||
| Malaria ( Plasmodium falciparum ) | X | X | X | X | X | |
| Toxoplasmosis | X | |||||
* Rare in other geographic locales.
Protozoa
Malaria
Epidemiology
Malaria is endemic throughout the tropical areas of the world; one half of the world’s population lives in areas where malaria occurs. From a global perspective, there are approximately 300 to 500 million infections per year, resulting in approximately 1 million deaths ( Fig. 13-1 ). Most of these deaths occur in children.

Etiology
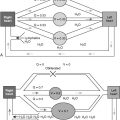
The Anopheles mosquito transmits the parasite ( Fig. 13-2 ). Various Plasmodium species— Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae —are responsible for human malaria. Of these four, only P. falciparum causes pulmonary disease. The disease onset is almost always within 1 year of exposure.

Clinical Manifestations
Shortness of breath and cough are the main respiratory symptoms in conscious patients; some also report chest tightness. Dyspnea often starts abruptly and progresses rapidly over a few hours, causing life-threatening hypoxia in patients with falciparum malaria.
Pathogenesis
The pathogenesis of malarial lung disease is attributed to a diffuse alveolar injury resulting in a capillary leak syndrome and acute pulmonary edema. This noncardiogenic pulmonary edema is associated with normal or low capillary wedge pressures without evidence of left ventricular dysfunction. Hypoalbuminemia and high-level parasitemia are risk factors associated with the development of pulmonary disease. Pathologic findings may include features of acute respiratory distress syndrome in some individuals; this is thought to be secondary to sequestered malaria parasites in the lung, inducing an inflammatory cascade. This mechanism mainly involves cytokines, neutrophils, and endothelial adhesion molecules. Substantial evidence implicates the neutrophil as central to the pathogenesis of microvascular lung injury, which results in increased capillary permeability and subsequent pulmonary edema.
Diagnosis
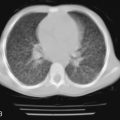
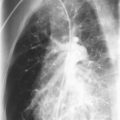
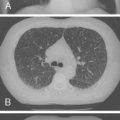
Radiographic and computed tomography (CT) findings may suggest noncardiogenic pulmonary edema. Pleural effusion, diffuse interstitial edema, and lobar consolidation may be seen. Laboratory assessments may show anemia and leukocytosis. A peripheral thick blood smear has high sensitivity for detecting parasitemia. A peripheral thin blood smear is better for species differentiation and staging of parasite development in P. falciparum .
Treatment
Effective treatment strategies for falciparum malaria depend on the pattern of parasite drug resistance in the geographic area where the infection is acquired and the severity of disease ( Table 13-2 ). The website for the Centers for Disease Control and Prevention should be reviewed to identify areas of chloroquine resistance. Severe malaria, such as that complicated by pulmonary disease, warrants therapy with parenteral medications. In addition to patient management in a critical care unit, the mortality rate is high. For physicians in nonmalarial areas, malaria always should be considered in the differential diagnosis of a sick patient who has traveled to a malaria-endemic area.
| INFECTION | DRUG | PEDIATRIC DOSE | ADULT DOSE |
|---|---|---|---|
| Malaria ( Plasmodium falciparum —in areas of chloroquine resistance) | |||
| Oral ( only in uncomplicated or mild disease) | |||
| Drugs of choice | Atovaquone/proguanil | <5 kg: not indicated | 2 adult tabs bid or |
| 5–8 kg: 2 pediatric tabs once daily × 3 days | 4 adult tabs once daily × 3 days | ||
| 9–10 kg: 3 pediatric tabs once daily × 3 days | |||
| 11–20 kg: 1 adult tab once daily × 3 days | |||
| 21–30 kg: 2 adult tabs once daily × 3 days | |||
| 31–40 kg: 3 adult tabs once daily × 3 days | |||
| 40 kg: 4 adult tabs once daily × 3 days | |||
| or | |||
| Quinine sulfate | 30 mg/kg/day in 3 doses × 3–7 days | 650 mg q8h × 3–7 days | |
| plus | |||
| Doxycycline | 4 mg/kg/day in 2 doses × 7 days | 100 mg bid × 7 days | |
| or plus | |||
| Tetracycline | 6.25 mg/kg qid × 7 days | 250 mg qid × 7 days | |
| or plus | |||
| Clindamycin | 20 mg/kg/day in 3 doses × 7 days | 20 mg/kg/day in 3 doses × 7 days | |
| Alternatives | Mefloquine | 15 mg/kg followed 12 hr later by 10 mg/kg | 750 mg followed 12 hr later by 500 mg |
| Artesunate | 4 mg/kg/day × 3 days | 4 mg/kg/day × 3 days | |
| plus | |||
| Mefloquine | 15 mg/kg followed 12 hr later by 10 mg/kg | 750 mg followed 12 hr later by 500 mg | |
| Malaria ( P. falciparum— chloroquine-susceptible) | |||
| Oral | |||
| Drug of choice | Chloroquine phosphate | 10 mg base/kg (maximum 600 mg base), then 5 mg base/kg at 24 hr and 48 hr | 1 g (600 mg base), then 500 mg (300 mg base) 6 hr later, then 500 mg (300 mg base) at 24 hr and 48 hr |
| Parenteral (all Plasmodium ) | |||
| Drugs of choice | Quinidine gluconate | 10 mg/kg loading dose (maximum 600 mg) in normal saline over 1–2 hr, followed by continuous infusion of 0.02 mg/kg/min until PO therapy can be started | 10 mg/kg loading dose (maximum 600 mg) in normal saline over 1–2 hr, followed by continuous infusion of 0.02 mg/kg/min until PO therapy can be started |
| or | |||
| Quinine dihydrochloride | 20 mg/kg loading dose in 5% dextrose over 4 hr, followed by 10 mg/kg over 2–4 hr q8h (maximum 1800 mg/day) until PO therapy can be started | 20 mg/kg loading dose in 5% dextrose over 4 hr, followed by 10 mg/kg over 2–4 hr q8h (maximum 1800 mg/day) until PO therapy can be started | |
| Alternative | Artemether | 3.2 mg/kg IM, then 1.6 mg/kg daily × 5–7 days | 3.2 mg/kg IM, then 1.6 mg/kg daily × 5–7 days |
| Ascariasis ( Ascaris lumbricoides —roundworm) | |||
| Drug of choice | Albendazole | 400 mg once | 400 mg once |
| or | |||
| Mebendazole | 100 mg bid × 3 days or 500 mg once | 100 mg bid × 3 days or 500 mg once | |
| or | |||
| Ivermectin | 150–200 μg/kg once | 150–200 μg/kg once | |
| Hookworm ( Ancylostoma duodenale, Necator americanus ) | |||
| Drug of choice | Albendazole | 400 mg once | 400 mg once |
| or | |||
| Mebendazole | 100 mg bid × 3 days or 500 mg once | 100 mg bid × 3 days or 500 mg once | |
| or | |||
| Pyrantel pamoate | 11 mg/kg/day(maximum 1 g) × 3 days | 11 mg/kg/day (maximum 1 g) × 3 days | |
| Strongyloidiasis ( Strongyloides stercoralis ) | |||
| Drug of choice | Ivermectin | 200 μg/kg/day × 2 days | 200 μg/kg/day × 2 days |
| Alternatives | Albendazole | 400 mg bid × 7 days | 400 mg bid × 7 days |
| or | |||
| Thiabendazole | 50 mg/kg/day in 2 doses × 2 days (maximum 3 g/day) | 50 mg/kg/day in 2 doses × 2 days (maximum 3 g/day) | |
| Amebiasis ( Entamoeba histolytica ) | |||
| Severe intestinal or extraintestinal disease | Metronidazole | 30–50 mg/kg/day in 3 doses × 7–10 days | 750 mg tid × 7–10 days |
| or | |||
| Tinidazole | 50 mg/kg/day (maximum 2 g) × 5 days | 2 g once daily × 5 days | |
| In treating extraintestinal disease follow with | Iodoquinol | 30–40 mg/kg/day (maximum 2 g) in 3 doses × 20 days | 650 mg tid × 20 days |
| or | |||
| Paromomycin | 25–35 mg/kg/day in 3 doses × 7 days | 25–35 mg/kg/day in 3 doses × 7 days | |
| Alternative | Diloxanide furoate | 20 mg/kg/day in 3 doses × 10 days | 500 mg tid × 10 days |
| Toxocariasis (visceral larva migrans) | |||
| Drug of choice | Albendazole | 400 mg bid × 5 days | 400 mg bid × 5 days |
| or | |||
| Mebendazole | 100–200 mg bid × 5 days | 100–200 mg bid × 5 days | |
| Echinococcosis ( Echinococcus granulosus —hydatid cyst) | |||
| Drug of choice | Albendazole | 15 mg/kg/day (maximum 800 mg) × 1–6 mo | 400 mg bid × 1–6 mo |
| Patients may benefit from surgical resection or percutaneous drainage of cysts | |||
| Echinococcosis ( Echinococcus multilocularis ) | Surgical excision is only available means of cure for this infection. In nonresectable cases, treatment with albendazole or mebendazole is recommended | ||
| Paragonomiasis ( Paragonimus westermani —lung fluke) | |||
| Drug of choice | Praziquantel | 75 mg/kg/day in 3 doses × 2 days | 75 mg/kg/day in 3 doses × 2 days |
| Alternative | Bithionol | 30–50 mg/kg on alternate days × 10–15 doses | 30–50 mg/kg on alternate days × 10–15 doses |
Amebiasis
Etiology and Epidemiology
Approximately 1% of the world’s population is infected with Entamoeba histolytica. After malaria and schistosomiasis, amebiasis is the third most common cause of mortality from parasitic diseases. Although pleuropulmonary disease in E. histolytica infection is uncommon, it can occur in 15% of individuals with an amebic liver abscess.
Pathogenesis
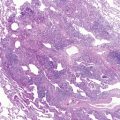
After a host ingests cysts in contaminated food or drink, pathogenic amebic trophozoites invade the colonic wall and disseminate hematogenously to the liver ( Fig. 13-3 ). Less commonly, infection may occur after aspiration. Disease onset may be either acute or chronic. Diarrhea is frequently absent. As an amebic liver abscess enlarges, adhesions may develop between the liver surface and the diaphragm. A sterile sympathetic pleural effusion may develop above the abscess. Alternatively, infection may spread through lymphatics or via the bloodstream and extend through the diaphragm after abscess rupture with empyema formation. The rupture frequently evokes severe pain in the right chest, back, or shoulder. Fever, hypoxemia, and circulatory collapse may follow. Classic “anchovy paste” can be obtained from an amebic hepatic abscess or from expectorate that has traveled through a hepatobronchial fistula.