FIG. 8.1 ● Components and resources to ensure quality services along the breast imaging continuum.
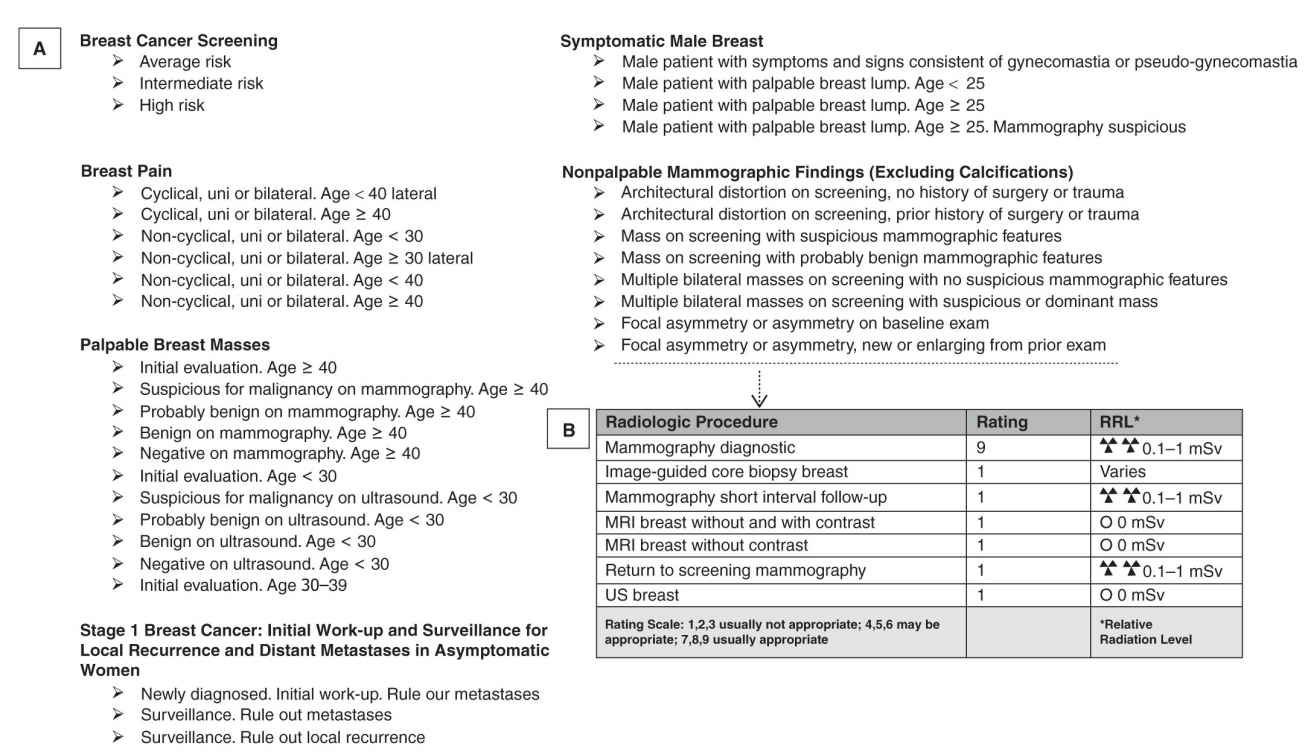
FIG. 8.2 ● A: List of the ACR appropriateness criteria (ACR AC) in breast imaging. B: Example of ratings for radiologic procedures for initial work-up of new or enlarging nonpalpable focal asymmetry. (Adapted from the ACR AC: https://acsearch.acr.org/list.)
APPROPRIATE IMAGE ACQUISITION AND INTERPRETATION
The ultimate goal of mammography is to identify breast cancer at earlier stages of the disease, which with advancement in treatments has been shown to have good prognosis and decreased mortality.12 Since its introduction as an imaging technique of the breast, the radiology community realized that ensuring the quality of image acquisition and interpretation of mammography was essential for its success. Improving quality has the potential of decreasing missed cancers (false negative) as well as decreasing unnecessary call backs (false positives).13 The ACR was at the forefront of these quality initiatives and, in 1987, initiated the ACR voluntary Mammography Accreditation Program (MAP).14 Initially, participation in this program was encouraged and not mandated. However, soon it became standard of care and even a requirement for health insurance reimbursement in some states.15 The MAP program addressed four categories of quality assurance (QA): equipment specifications, equipment performance, facility QA procedures, and personnel qualifications. The variability in adoption and implementation of the MAP program by various states was the impetus for the Mammography Quality and Standards Act (MQSA), which was enacted by Congress in 1992 and reauthorized and amended in 1998 and 2004, respectively. This legislation mandated that mammography facilities have to be certified by the federal government. The specifics of this mandate, primarily based on the MAP program, nevertheless further standardized the process of certification and accreditation. The inspection and certification of mammography facilities was delegated to the Department of Health and Human Services (HHS), which in turn delegated the task of annual certification of mammography facilities to the U.S. Food and Drug Administration (FDA). The MQSA legislation also mandated oversight and accreditation of mammography facilities by an FDA-approved accreditation body; this is typically on a 3-year cycle. Given the experience that the ACR had in quality accreditation process, it was intuitively chosen as the primary FDA-approved accreditation body for mammography facilities. The Arkansas, Iowa, and Texas departments of state health services were also approved as accreditation bodies for facilities in their respective states.16
THE COMPREHENSIVE ACCREDITATION IN BREAST IMAGING
Initially, the MQSA was enacted to ensure performance of quality mammographic imaging and interpretation. However, the rapid technologic advancement in breast imaging necessitated that the Breast Imaging Quality Assurance Programs expand to include accreditation programs for breast ultrasound, ultrasound- and stereotactic-guided breast procedures, and breast MRI. These additional accreditation programs are not mandated by MQSA; however, they are conducted through the ACR, and enrollment in these programs is encouraged to maintain quality of breast imaging services. Currently, a designation of ACR Imaging Center of Excellence is provided to imaging centers that not only pass the mandated ACR MAP, but also voluntarily apply to and pass the accreditation programs for other provided breast imaging services. In the next few sections, we will provide a general overview of the accreditation programs for each of the breast imaging technologies
Mammography Accreditation Programs
To perform mammography, imaging facilities are required by law to be certified and inspected by the FDA, and accredited by an accrediting body. As we review the specifics of each of these accreditation programs, we will identify overlap in some of the requirements. However, in combination, they cover all aspects of quality and safety in performance and interpretation of mammography.
FDA Certification
Timing
• Initial certification
any time new mammography equipment is installed or equipment replaced.
• Yearly renewal for the already certified facilities and equipment
Components
A separate certification process has to be initiated for each clinic or imaging center that performs mammography, even when belonging to the same organization or radiology group. Of note, the FDA certification is a collective certification for the individual geographic locations or clinics.
The certification process involves the following:
A. Submitting an application for certification (initial or renewal) that includes:
a. The name and qualifications of the designated lead interpreting radiologist who is ultimately responsible that the quality control (QC) tests and measures have been met
b. The name and qualifications of the designated lead mammography technologist who oversees the performance of the QC tests
c. The name and qualifications of the designated lead physicist who performs the required annual tests and produces the annual report
d. The information on each mammography unit used for clinical imaging at that location (vendor, make, and model)
e. Types and number of mammographic examinations performed (screening versus diagnostic) in the last 12 months
f. The names and qualifications (educational background, training, and professional experience) of all the radiologists interpreting the mammographic examinations
g. The names and qualifications of all the technologists performing the mammography examinations
B. Application fees for the facility and each mammography unit
C. A physical site visit by a designated state or FDA mammography inspector. During this site visit, the inspector will review:
a. Records of completing the recommended QC tests for each mammography unit in that facility for the past 12 months
b. Records of initial certification and maintenance of certification requirements for each radiologist interpreting mammographic examinations at that facility
c. Records of initial certification and maintenance of certification requirements for each mammography technologist acquiring mammographic examinations
d. Records of the medical outcomes audit
Key Points Every Radiologist Should Know Regarding Quality Control Program in Mammography
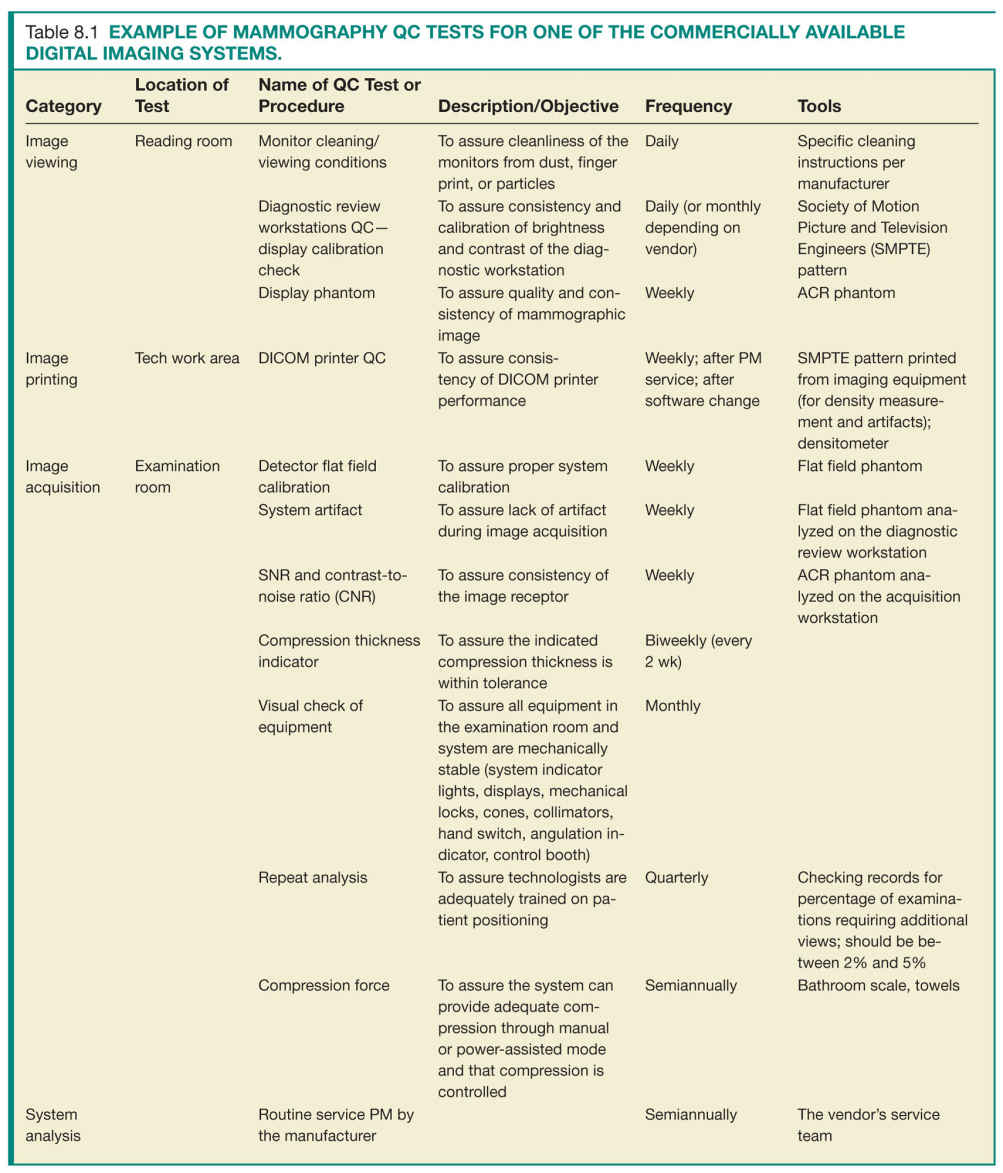
• There are some general QC requirements that are common between the different imaging acquisition techniques (screen-film versus digital imaging) and between different commercially available manufacturers, while other requirements or timing of tests will be specific to individual systems (Table 8.1).
• Manufacturers will routinely provide technologists training on the specifics of QC tests, with the installation or upgrade of their equipment. Additionally, they will provide detailed QC manuals and checklists that can guide the technologists during routine QC tests.
• The role of the lead mammography technologist is to perform mammography or identify key technologists who will be delegated the specific QC tests and checklists as well as to ensure that all the QC tests are performed according to the timing and technique specified by individual manufacturers and are well documented. Prior studies have estimated that the time involvement in maintaining the QC tests is approximately 160 hours per year.17
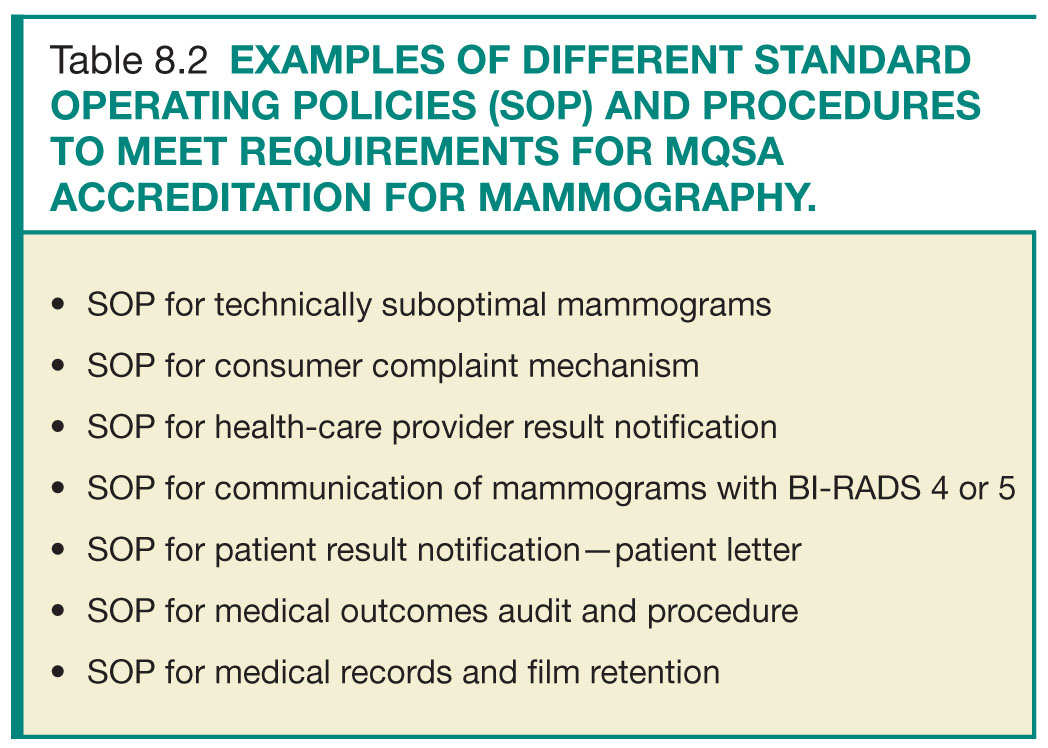
• The role of the lead interpreting physician is to work in close collaboration with lead mammography technologist in oversight of the QC process and documentation, in creation of QA policies, procedures, and workflow strategies to fulfill the MQSA regulations (Table 8.2), and to identify areas for workflow improvement. Additionally, one of the key roles of the lead interpreting radiologists is to closely review the medical outcomes audit for the entire practice and for individual radiologists and to compare these metrics to national benchmarks. This is crucial for identifying trends or outlying performers and facilitating practice improvement measures. This will further be discussed in the Medical Outcomes Audit section.
Personnel Qualifications
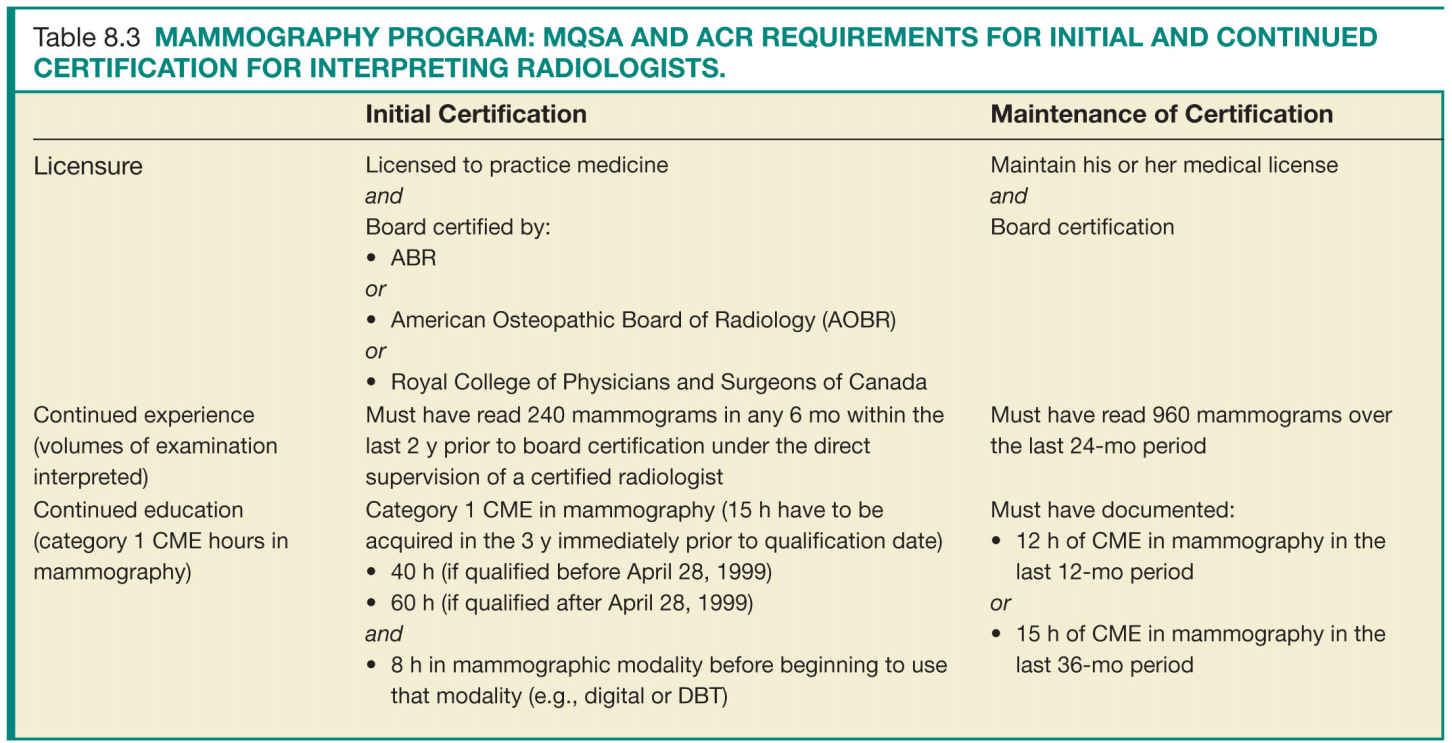
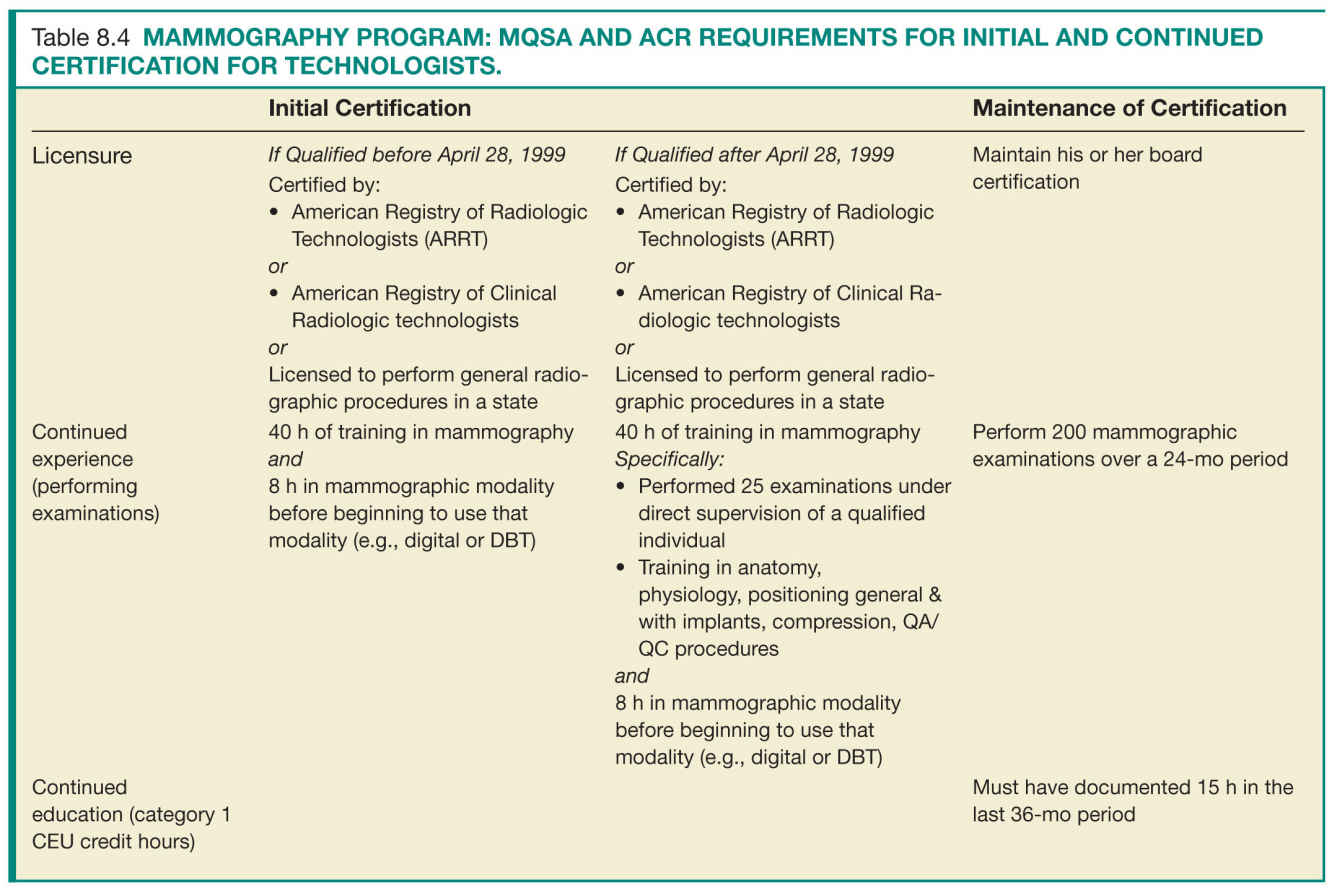
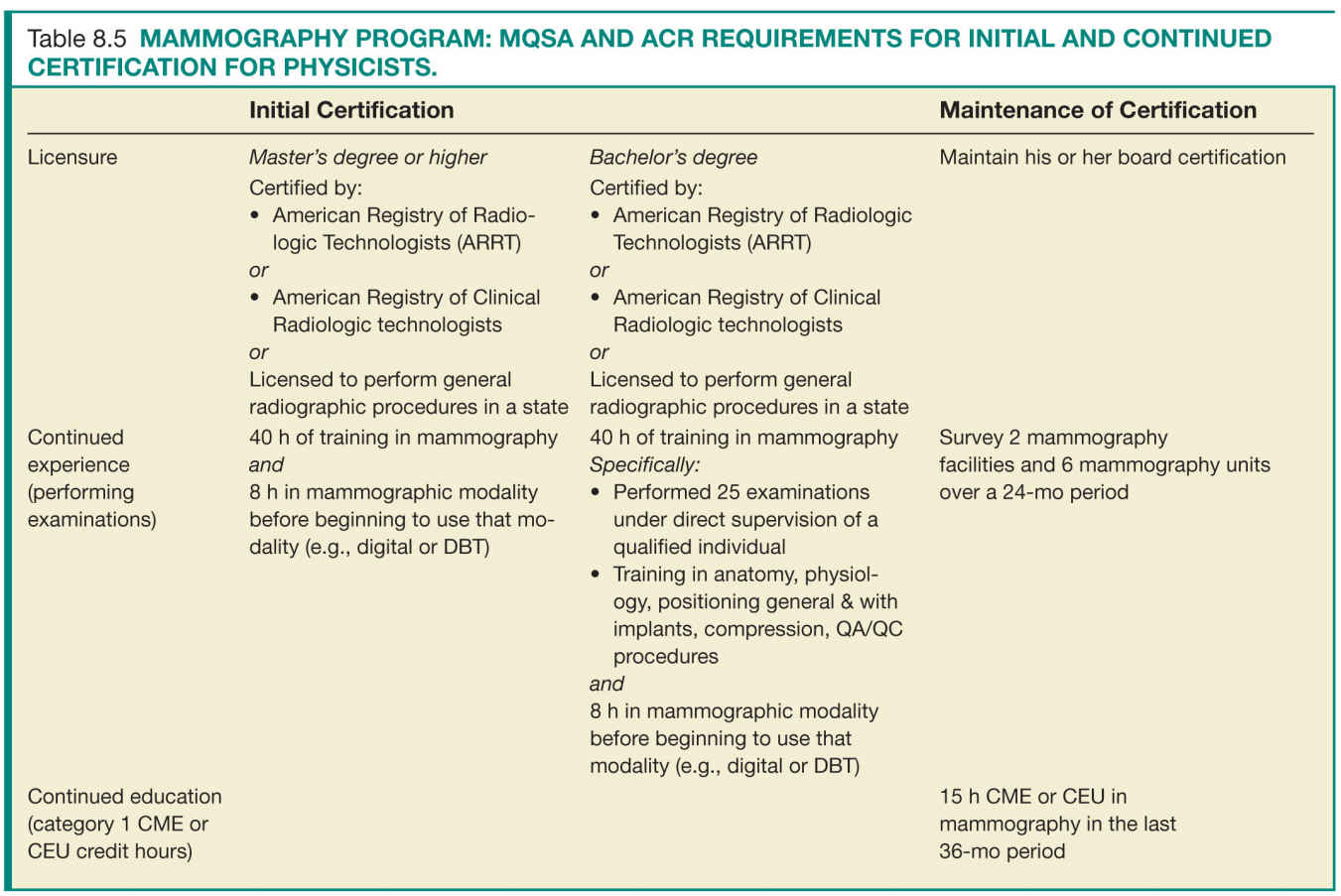
For the success of a QA program, the individuals involved in acquiring and interpreting the mammographic examinations have to meet minimal requirements that ensure their ability to perform their delegated tasks up to predefined quality standards. There are specific requirements for initial certification and maintenance of certification. Tables 8.3 to 8.5 list the initial certification and maintenance of certification requirements for radiologists, technologists, and physicists in a mammography facility.
The designated FDA inspector who conducts the onsite visit also has to meet certain certification requirements; however, this is beyond the scope of this chapter.
For radiologist and technologist who rotate between the various satellite clinics that belong to the same organization or breast imaging practice, a travelling book with copies of the initial certification and maintenance of certification records is required at each imaging facility during the inspection visit.
Equipment Specifications and Physicist Mammography Equipment Evaluations
There are specific hardware and software requirements for mammography equipment that the FDA mandates for clinical care and can be reviewed at http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/default.htm.
Routine preventative maintenance (PM) for each mammography unit by qualified service engineers is also a requirement. Reports of these PM checks as well as any deficiencies identified and corrective action should be kept for records.
Mammography equipment evaluation (MEE) is an extensive process performed by an MQSA qualified physicists, and assures that the equipment installed meets FDA technical specifications and its performance passes the FDA QA program (the medical physicist’s survey).
The MEE evaluation must be conducted after:
1. A new unit is installed
2. A previously accredited unit moved from one location to another
3. Major repair or upgrades
The Medical Physicist’s Survey: These are checklists of QA tests that are conducted by the lead physicists at initial certification, then at least annually or after every major equipment repair. After completion of this inspection, the physicist generates a report indicating that the unit passed all QC tests and is eligible for clinical care. This constitutes part of the MEE and is included with certification paperwork and kept with the QC documents (www.fda.gov/RegulatoryInformation/Guidances/ucm094405.htm).
Additional requirements for MQSA certification include the following:
• Facilities must ensure that final mammography reports are sent to the referring physicians as well as a summary of these results sent to the patient in lay terms as soon as possible, no longer than 30 days.
• Facilities must keep patients’ prior mammograms as part of their permanent medical records somewhere between 5 and 10 years.
ACR Accreditation
• The majority of mammography facilities across the United States will need to be accredited by the ACR. The exceptions are the facilities in Texas, Arkansas, or Iowa, which can be accredited by their respective state departments of HHS.
• The ACR certification process has to be initiated for each clinic or imaging center that performs mammography, even when belonging to the same organization or radiology group, and it entails the certification of each imaging unit within that location.
• The ACR accreditation for mammography adheres to the same requirements as the FDA certification. In addition, it assures the quality of images acquired at each facility by reviewing an example of the facility’s clinical images, which are submitted at the time of certification.
• Although onsite visit is not mandatory for ACR accreditation, the ACR will conduct onsite survey to random sample of mammography facilities across the United States. If a facility is chosen for a random site visit, it will be notified in advance. The survey team will include the ACR radiologist, medical physicist, and ACR staff technologist, and items that will be reviewed during their visit include:
1. Documentation of personnel qualifications
2. Documentation of the QA program
3. Documentation of the policies and procedures
4. Mammography images and reports from clinical cases
Timing
• Initial certification any time new mammography equipment is installed or equipment replaced.
• Renewal process for the already certified equipment. This is conducted on a 3-year accreditation cycle. The ACR will send a notification of renewal 8 months prior to the expiration of the accreditation cycle.
Components
The ACR accreditation is a two-step process:
First, the application for equipment certification can be filled online or sent by mail. The information included in the initial application is as follows:
A. Basic facility information, including detailed equipment information and personnel information
B. The detailed medical physicist’s MEE; summary report detailing that all the equipment meets FDA specifications and have passed all required FDA QA tests, as detailed in the FDA Certification section.
C. Certification fees
Second, if all the initial application paperwork is complete, the ACR will send the facility access to online testing package. This package will have details on additional paperwork, additional forms to be completed, and instructions on how to send and label copies of actual clinical images that are acquired by the facility after the initial application approval. Once the testing package paperwork and images are completed, they should be sent back to the ACR within 45 days of receipt of the testing package.
The testing package that is sent back to the ACR will include the following:
• Personnel information and qualifications (physicians, technologists, physicist)
• Facility policies and QA procedures, including reporting mechanism
• Medical outcomes audit
• QC results
• Clinical images
Personnel Qualifications
Facilities should have documentation of the requirements for initial and continued certification for mammography for all interpreting radiologists, technologists, and medical physicists who follow the same guidelines and requirements as the FDA certification (Tables 8.3 to 8.5).
Equipment Specifications and Quality Control
The equipment specifications and QC procedures required for ACR accreditation adhere to the same requirements discussed in the FDA Certification section for mammography. Part of the ACR application packet is submitting the FDA renewal letter for the facility that ensures adherence to these requirements.
ACR Phantom Image
The ACR phantom is used by mammography technologists and physicists to conduct their QC testing. The phantom simulates a 4.2-cm compressed breast of average density. It includes various sized semi-radiopaque wax inserts, fibers (six), specks groups (five), and masses (five).
This phantom is used by technologists to assess optical density, contrast uniformity, and image quality of the imaging system to fulfill the MQSA and ACR mandated QC tests. The phantom is imaged on the mammography equipment being tested. In digital systems, the image of the ACR phantom is reviewed on the digital monitor used in clinical interpretation. For screen-film system, the film is printed and read on the view box used for clinical interpretation. To pass the phantom test, the image must show a minimum of four fibers, three speck groups, and three masses (Fig. 8.3). And, the average glandular dose should not exceed 300 mrad (3 mGy).
For ACR accreditation, a copy of the phantom image is sent with the application packet and will be reviewed by two ACR physicists.
Quality Assurance
Procedures and Policies
These are documents that detail the facility workflow strategies and steps taken to assure quality of the provided clinical services in adherence to the MQSA regulations. They are typically created by the lead interpreting physician and lead QC technologist taking into account the facility’s resources (Table 8.2). These constitute a great opportunity for workflow improvement projects, and should be routinely reassessed and updated for optimal efficiency and performance.
The Medical Outcomes Audit
Medical outcomes audit of mammography facilities is a significant component of the comprehensive mammography QA program. The process of medical audit has four major components: an initial step of accurate data accrual, a second step of calculations of derived data, a third step of reviewing and analyzing the outcomes data and comparing it to national benchmarks, and lastly, the fourth step of introducing practice improvement measures (Fig. 8.4). The initial three steps can then be repeated for assessing the impact of the introduced practice improvement measure.
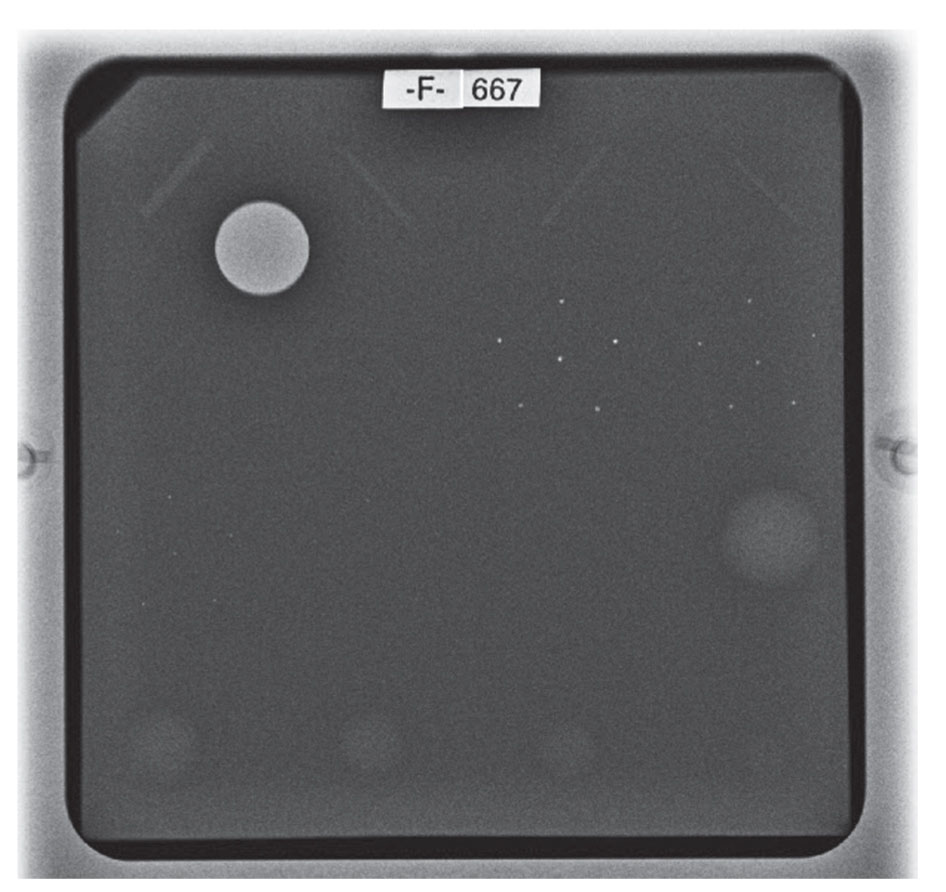
FIG. 8.3 ● Digital image of the ACR phantom: technically adequate imaging system with at least four (4) fibers, three (3) speck groups, and three (3) masses are identified.
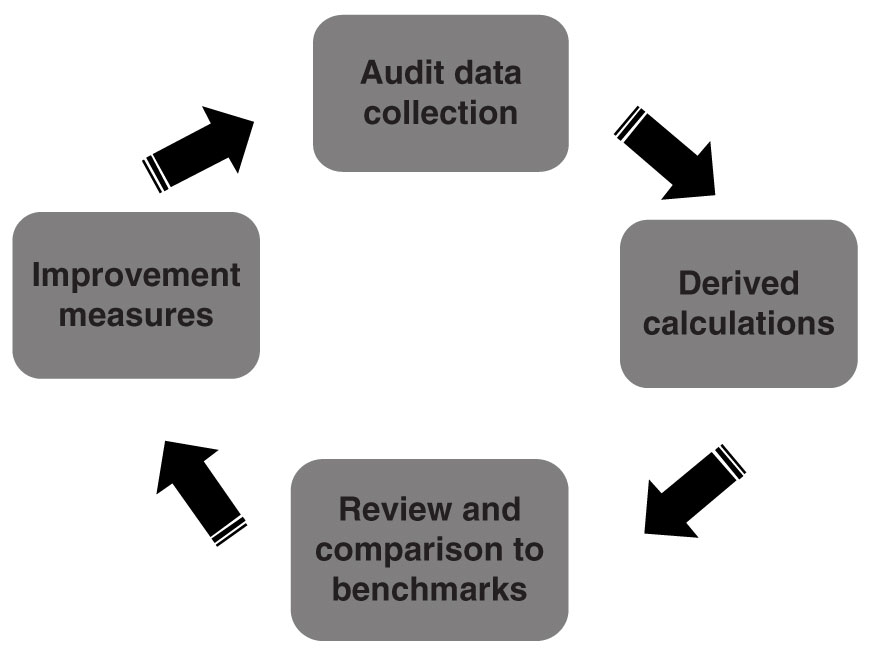
FIG. 8.4 ● Medical outcomes audit cycle.
For this comprehensive process to take place, breast imaging facilities must have a mammography medical outcomes audit program in place to follow positive mammography assessments and to correlate pathology results with the interpreting physician’s findings.18 The process of maintaining an accurate and meaningful medical audit program is lengthy and time consuming. Nonetheless, it is crucial in providing feedback to interpreting radiologists on their individual performance in detecting early cancers as well as their collective performance as a group in comparison to national standards with the ultimate goal of improved patient care.19–21
Step 1—Data accrual: The initial step of data accrual had historically been done manually. However, after the introduction of structured mammography reporting systems, this process has become semiautomated. These reporting systems typically have a medical outcomes audit package that tracks the total number of examinations based on indication, the interpreting radiologist, and the BI-RADS assessment category of each examination. Additionally, the majority of these systems will have a pathology reporting interface that allows manual entry of pathology results for each biopsy recommended and performed by the facility. These results (positive or negative) are in turn attributed to the interpreting physician for each of the screening and diagnostic mammogram, a step required in the process of outcomes calculation. Some of the newer commercially available systems also have the capability of linkage to the patients’ EMRs and automating the process of importing pathology results, thus eliminating the manual entry of pathology data and potentially avoiding human errors and saving resources.
Each facility should have a QA process in place for follow-up on patients with mammograms coded as BI-RADS 4 or 5 that do not schedule or show up for biopsy at their institution. From a safety and quality standpoint, this process is to ensure that every attempt has been made to avoid missing cancers due to patient noncompliance, through contacting the patient’s referring physician. Additionally, if the patient decides to receive care outside of the initial imaging facility, then this process would allow for the collection of pathology results for outcomes calculation.
Step 2—Calculation of derived data: Guidance on the methods of calculation of the medical outcomes audit is included in the BI-RADS atlas.22 These calculations can be automatically generated through the structured reporting systems that generate comprehensive reports that include all the collected and calculated data recommended for accreditation. These reports can be performed on aggregate facility numbers or for individual radiologists.
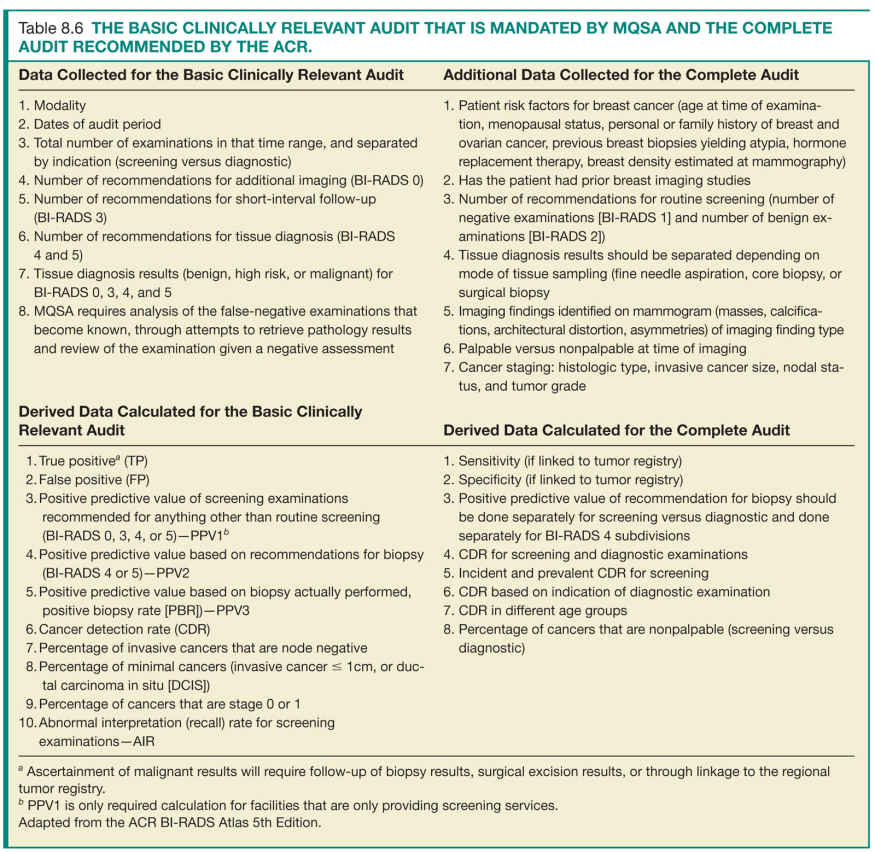
Under MQSA regulations, conducting a minimal audit is required by facilities for accreditation purposes. However, the ACR recommends mammography facilities to conduct a more detailed audit to accurately assess the clinical performance of its interpreting physicians (Table 8.6).
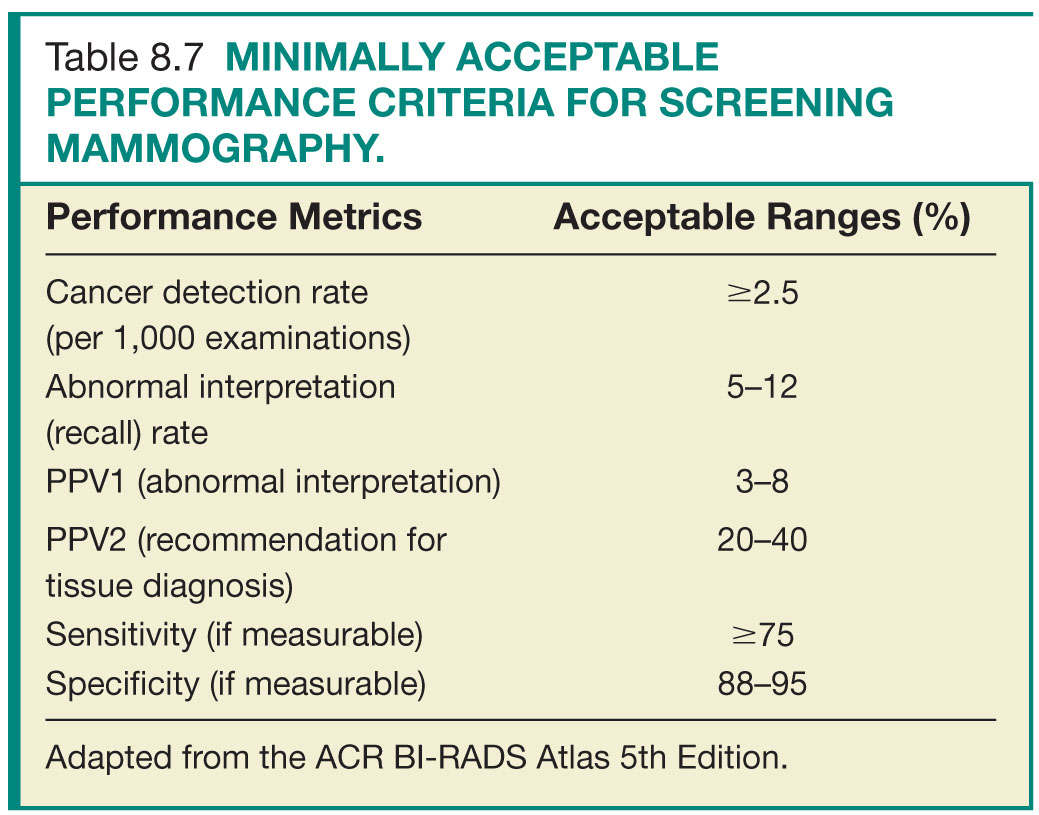
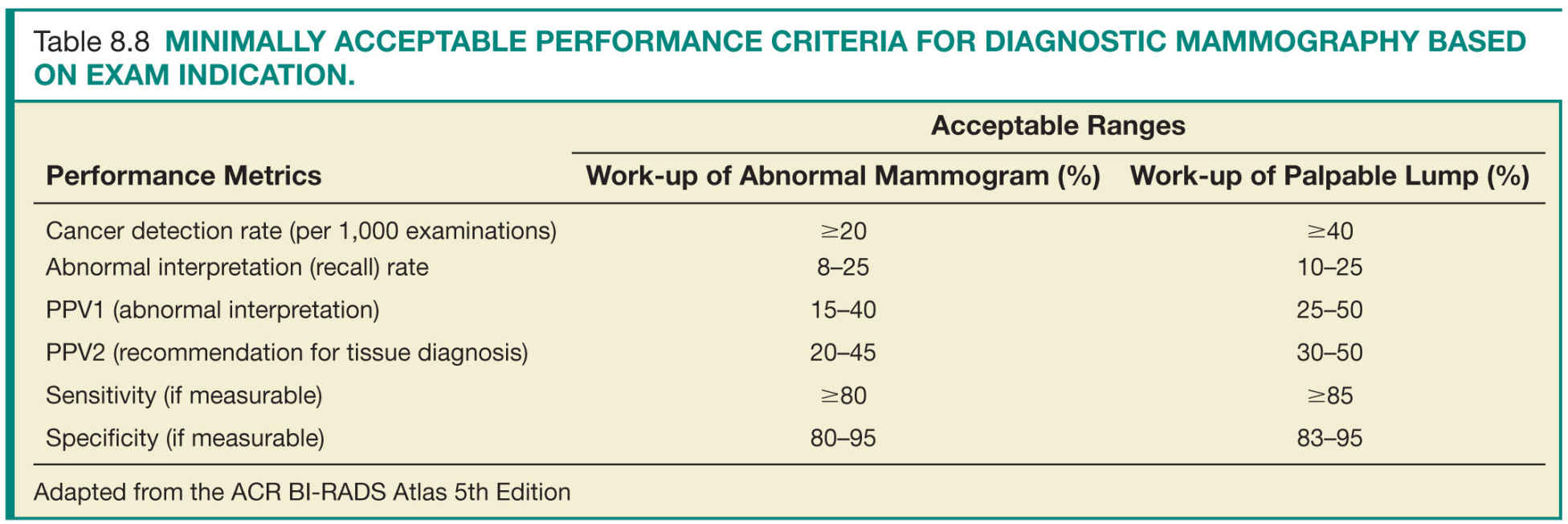
Step 3—Data review and analysis: Review of the medical outcomes audit is the responsibility of the lead interpreting physician. This entails review of the aggregate facility and individual radiologist data. Data analysis and comparison to national benchmarks (based on data from national data repositories as well as modeling studies) identifies areas for potential improvement (Tables 8.7 and 8.8). Most importantly, as most of the statistical calculations of the derived data are based on correlation between the prospectively assigned BI-RADS assessment categories and the clinical outcome of mammograms, the interpreting radiologists should have a clear understanding and strong adherence to the ACR guidelines and the ACR BI-RADS terminology, assessment categories, and management recommendations.23
Step 4—Practice improvement measures: Through this comprehensive medical review process, areas of deficiencies should be addressed by the lead interpreting physician and practice improvement measures should be put in place. Some of the interventions that could improve performance recommended in previously published studies include additional education for technologists or interpreting radiologists, ensuring availability of prior comparisons, routine review and group discussion of false-negative cases, and workflow changes to allow uninterrupted reading sessions.24–28
National Mammography Database
Despite the presence of the BI-RADS atlas as a comprehensive guide to radiologists, significant variability in practices still exists. Prior studies documenting and examining these variabilities attributed them partly to subspecialty training, years of experience, volumes of yearly interpreted examinations, and degree of adherence to BI-RADS recommendations.29–34 And, since then, there has been a consorted effort by the radiology community to collect data on radiologists’ performance through large national data repositories. These data repositories allowed for multi-institutional studies that focused on establishing national benchmarks and interpretative criteria, to minimize the variability in practices among radiologists and improve performance.20,35–37 These set of performance metrics, as detailed in the ACR BI-RADS atlas,22 are well-established quality measures in qualifying the collective success of a mammography practice as well as providing feedback to interpreting radiologists on their individual performance in comparison to national standards.18–21,38
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree