Chapter 14 Quality management in radiotherapy
Introduction – what is quality?
There are many different, well-documented theories and approaches to quality and quality management [1]. Approaches to quality have seen a shifting emphasis and have evolved through the following stages:
1. inspection and remedial action
2. identifying improvements and taking steps to prevent mistakes occurring
This is reflected in the history of quality in radiotherapy [2], such that the interpretation of quality has widened and can now be applied to every aspect of the service, incorporating many local, professional and national standards. It is not possible to cover every aspect of quality in detail in a single chapter. This chapter aims to provide an overview of quality and quality management and their significance to radiotherapy, outline the requirements of the international quality standard BS EN ISO 9001, signpost readers to further information on a number of quality initiatives and promote an integrated approach to quality management in UK Oncology Centres.
Quality is the totality of features and characteristics of a product or service that bear on its ability to satisfy stated or implied needs [3].
Another often used term is quality control (QC): ‘the part of quality management focused on fulfilling quality requirements, [4].
Quality assurance (QA) refers to ‘the part of quality management focused on providing confidence that quality requirements will be fulfilled’ [4] or: ‘a systematic process of verifying that a product or service is meeting specified requirements’ [5].
Quality management (QM) is ‘the coordinated activities to direct and control an organization with regard to quality’ [4]. The underpinning philosophy, to get the process and the service right first time, applies to all parts of the organization, involving managing all functions and activities to achieve quality.
A quality management system (QMS) is a management system to direct and control an organization with regard to quality [4].
• identification of clear requirements or standards
• identification of clear processes, broken down into identifiable steps
• a focus on the prevention of problems
The steps to achieving any quality initiative must include the following stages:
The main aim of quality in radiotherapy is safe and effective treatment. In addition to ensuring that all treatments are optimized, a quality system should ensure that all possible measures are taken to prevent inappropriate exposures from occurring [6].
History of quality in radiotherapy
There are a number of well-documented radiotherapy incidents [7–12], which have occurred both in the UK and internationally (Table 14.1). These have led to hundreds of patients receiving incorrect doses of radiation which, in turn, may have led to the possibility of the treatment intent being compromised or causing unnecessary and distressing acute and late side effects of treatment. Subsequent outcomes in the UK have included key reports which have contributed to the development of quality management systems in radiotherapy.
Table 14.1 Chronology of quality development in radiotherapy in the UK
| Year | Event | Reference |
|---|---|---|
| 1988 | Exeter. Overdose, 207 patients affected | |
| 1988 | Thwaites Report: detailed root cause of Exeter incident. | [13] |
| DoH working party, chaired by Professor Bleehen, to investigate the application of quality assurance to radiotherapy to reduce the probability of incidents occurring in other centres. | ||
| 1982–1991 | Stoke. Underdose, 1045 patients affected | |
| 1991 | Bleehen Report: Quality assurance in radiotherapy (QART). Took the form of a quality standard and provided a formal system for managing quality in radiotherapy. It was based on the Quality Standard BS 5750, Part 2, 1987 (the predecessor of ISO 9001), a quality standard which was generic to a range of industries and services and thus, open to a degree of interpretation and translation. | [14] |
DoH funded two pilot sites, Bristol and Manchester: | ||
| 1994 | Stoke: report from investigation. | [15] |
| 1994 | Sample documents from two pilot sites published and DoH recommendation for all departments to develop their own quality management system (QMS) | [16] |
| 1994 | BS EN ISO 9001:1994 replaced BS5750 | |
| 2000 | BS EN ISO 9001:2000 replaced BS EN ISO 9001:1994 | [17] |
| 2006 | Incident at Beatson Oncology Centre | [18] |
| 2006 | Report into overexposure at Beatson | [19] |
| 2008 | Redacted Independent review of the circumstances surrounding a serious adverse incident | [12] |
• lack of communication, especially at staff group interfaces
• training, competency and supervision issues
• lack of up-to-date procedures, protocols and documentation
• no independent check/calibration
It is of the utmost importance that the radiotherapy community shares information and learns from past mistakes [6,20]. Departments should also search for, correct, monitor and learn from their own incidents. Departments open themselves up to risk if there are workload or time pressures or if there is an insufficiency in:
• attention to detail, alertness or awareness
• resources, e.g. qualified and well-trained staff
• coordination, communication and clear responsibilities [6].
Quality management systems
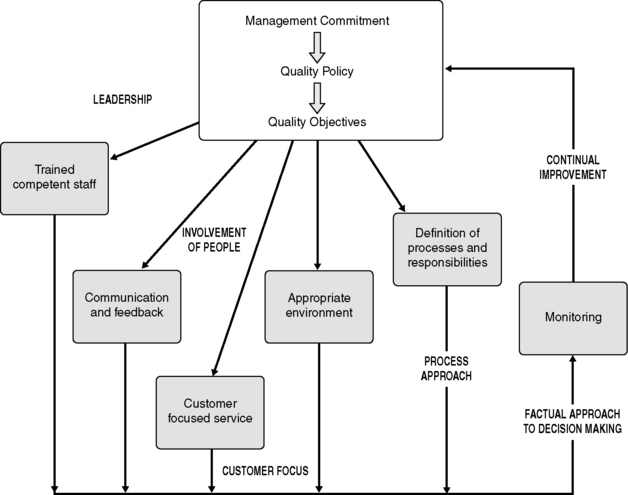
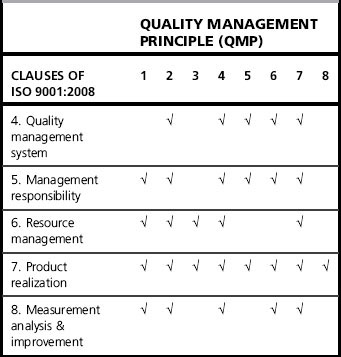
A quality management system (QMS) is a set of interrelated or interacting processes which provide a framework for an organization to manage its activities in order to achieve its quality policy and quality objectives. Management system standards, such as ISO 9000 (see below), provide a model to follow in setting up and operating a quality management system. The ISO 9001 requirements are based on eight quality management principles (QMP), the key elements of which are illustrated in Figure 14.1.
ISO 9000
• ISO 9000 Quality management systems – Fundamentals and vocabulary (last published 2005 [21])
• ISO 9001 Quality management systems – Requirements (last published 2008 [22])
• ISO 9004 Quality management systems – Guidelines for performance improvements (last published 2000 [23], due for republication 2009 [24] with a new focus on ‘sustained success’).
The ISO 9001:2008 [22] standard comprises nine clauses, listed in Box 14.1.
Clause 0, Introduction, describes a ‘process approach’ and compatibility with other management systems. Clause 1, Scope, specifies the type of organization that the standard is applicable to. Clause 2, Normative references, gives reference to the document ISO 9000:2005 [21], which is necessary for the application of the ISO standard. Clause 3, Terms and definitions, defines what particular terms, e.g. product, mean within the concept of ISO 9000. The remaining clauses, 4–8, contain the requirements against which departments are assessed.
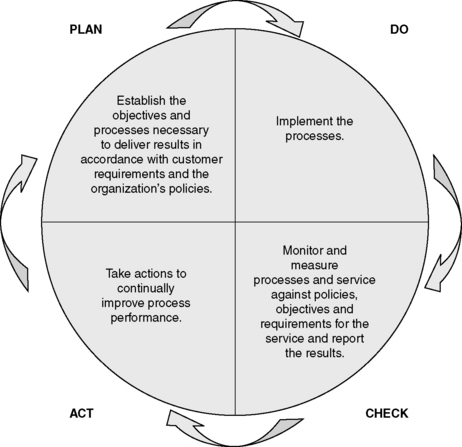
These requirements are based on the eight quality management principles outlined above. Table 14.2 illustrates the quality management principles which underpin the clauses of ISO 9001. The ISO 9001:2008 requirements are very much based on the Plan – Do – Check – Act cycle [22], as illustrated in Figure 14.2. Clauses 4–8 and their requirements are described further below, with the relevant quality management principles (QMP) indicated in brackets.
Clause 4 Quality management system (QMP 2, 4, 5, 6, 7)
1. control of documents (Clause 4)
2. control of records (Clause 4)
4. control of non-conforming product (Clause 8), e.g. complaints, clinical incidents
5. corrective action (Clause 8)
A QMS will also contain all or some of the following:
Control of all these documents includes regulating their development, approval, issue, identification, amendments, distribution, storage, security, obsolescence, retention and disposal. Similarly, records produced, such as training records, patient treatment records, equipment maintenance records and records of complaints and incidents, must be regulated for the duration of their life cycle. A record may be any document that provides evidence of activities performed. Records must be legible, identifiable and retrievable. A department must define what its quality records are, how they are stored, who has responsibility for them, how long they should be retained and the method of disposal. Departments must ensure they incorporate legislative [25, 26], national [27–29] and local requirements into their control of documents and records policies.
Clause 5 Management responsibility (QMP 1, 2, 4, 5, 6, 7)
Specific areas of management responsibility outlined in this clause include:
• evidence of management commitment
• determining, meeting and enhancing customers’ requirements
• an established quality policy which reflects the vision of the organization
• established quality objectives which are based on the quality policy and business objectives, and aim to fulfill the needs of the customers
• management review meetings to review the effectiveness of the QMS and the department’s performance against objectives, and to identify further improvements
• ensuring the availability and effective allocation of the resources required to fulfil the department’s objectives.