Radiation Carcinogenesis
▪ DETERMINISTIC AND STOCHASTIC EFFECTS
If cellular damage occurs as a result of radiation and it is not adequately repaired, it may prevent the cell from surviving or reproducing or it may result in a viable cell that has been modified, that is, suffered a change or mutation that it retains as a legacy of the radiation exposure. The two outcomes have profoundly different implications for the person of whom the cell is a part.
Most organs or tissues of the body are unaffected by the loss of a few cells; but if the number of cells lost is sufficiently large, there is observable harm, reflecting the loss of tissue function. The probability of such harm is zero at small radiation doses, but above some level of dose, called the threshold dose, the probability increases rapidly with dose to 100%. Above the threshold, the severity of harm also increases with dose. Effects such as these are said to be deterministic. A deterministic effect has a threshold in dose, and the severity of the effect is dose related. Radiation-induced cataracts and late tissue fibrosis are examples of deterministic effects.
The outcome is very different if the irradiated cell is viable but modified. Carcinogenesis and heritable effects fall into this category. If somatic cells are exposed to radiation, the probability of cancer increases with dose, probably with no threshold, but the severity of the cancer is not dose related. A cancer induced by 1 Gy is no worse than one induced by 0.1 Gy, but of course, the probability of its induction is increased. This category of effect is called stochastic, a word that has been given a special meaning in radiation protection but in general, just means “random.” If the radiation damage occurs in germ cells, mutations may occur that could cause deleterious effects in future generations. Again, there is probably no threshold and the severity of heritable effects is not dose related, although the probability of it occurring is.
The belief that stochastic effects have no dose threshold is based on the molecular mechanisms involved. There is reason to believe that even a single x-ray photon could result in a base change leading to a mutation that could cause cancer or a heritable defect. For this reason, it is considered prudent and conservative to assume that no dose is too small to be effective, although this can never be proved.
The two types of effects are summarized as follows:
Deterministic effect: severity increases with dose; practical threshold; probability of occurrence increases with dose (e.g., cataract).
Stochastic effect: severity independent of dose; no threshold; probability of occurrence increases with dose (e.g., cancer).
▪ CARCINOGENESIS: THE HUMAN EXPERIENCE
Cancer induction is the most important somatic effect of low-dose ionizing radiation. In sharp contrast to the case for the heritable effects of radiation (Chapter 11), risk estimates for leukemogenesis and carcinogenesis do not rely on animal data but can be based on experience in humans. There is a long history of a link between radiation exposure and an elevated incidence of cancer. Figure 10.1 is a beautiful photograph of Marie Curie and her daughter Irene, who are both thought to have died of leukemia as a result of the radiation exposure they received while conducting their experiments with radioactivity. Figure 10.2 is a photograph of the hand of a dentist in New York who held x-ray films in patients’ mouths for many years and who suffered malignant changes as a result. Quantitative data on cancer induction by radiation come from populations irradiated for medical purposes or exposed deliberately or inadvertently to nuclear weapons. Persons exposed therapeutically received comparatively high doses, and their susceptibility to the effects of radiation might have been influenced by the medical condition for which treatment was being given. Populations exposed to γ-rays and neutrons from nuclear weapons represent a wider cross section in terms of age and health and also include persons exposed to lower doses. In both cases, dose rates were high and exposure times brief.
There are a few groups of exposed persons to whom these generalizations do not apply. Examples include pitchblende and uranium miners who inhaled the radioactive gas radon and its progeny products over a prolonged period, patients injected with radium chloride or Thorotrast for medical purposes, and persons who ingested radionuclides while painting luminous dials on clocks and watches with paint containing radium. Hundreds of thousands of nuclear workers have been exposed occupationally, and useful cancer risk estimates have become available in recent years. Miners exposed to radon in the uranium mines are an excellent source of data on lung cancer.
The early human experience of radiation-induced cancer may be summarized as follows:
Skin cancer and leukemia were common in early x-ray workers, principally physicists and engineers who worked around accelerators before radiation safety standards were introduced.
Lung cancer was a frequent problem in pitchblende miners in Saxony, who dug out the ore from which radium was extracted. In the years following World War II, lung cancer also was noted in uranium miners in the central Colorado plateau. In both cases, the mines were poorly ventilated and there was a buildup of radon gas in the atmosphere of the mine; radon and its progeny were inhaled by the miners, depositing atoms of radioactive material in their lungs. The intense local α-radiation was responsible for inducing lung tumors. Bone tumors were observed in the radium dial painters. The painters were mostly young women who worked in factories in which the luminous dials on clocks and watches were painted with a special paint preparation containing radium. The workers dipped their brushes into the radium paint and used their tongues to shape the brushes into sharp points to paint the small dials on watches. As a result, some radium was ingested, which, because it is in the same group in the periodic table as calcium, was deposited in the tips of the growing bones. The intense α-radiation produced bone tumors. There is also history of bone tumors in people who, in the 1920s and 1930s, received injections of radium salts for the treatment of tuberculosis or ankylosing spondylitis.
An excess incidence of liver tumors was reported in patients in whom the contrast material Thorotrast was used. Thorotrast contains radioactive thorium, which, when deposited in the liver, produced a small incidence of liver tumors by α-radiation.
These early examples are interesting but largely anecdotal, although they did alert scientists to the danger of excessive radiation exposure. None of these examples involved situations that now constitute a public health hazard; these problems will never happen again, and the dosimetry in each instance is so uncertain that it is rarely possible to deduce any quantitative relationship between the dose of radiation involved and the tumor incidence.
More recent examples of the human experience with radiation-induced cancer and leukemia include the following:
The Japanese survivors of the atomic bomb attacks on Hiroshima and Nagasaki are the most important single group studied because of their large number, the care with which they have been followed, and the fact that people of all ages and both sexes received a wide range of doses. About 120,000 people have been followed carefully, of whom about 50,000 received doses in excess of 0.005 Sv. By 1998, there had been more than 17,000 cases of cancer, of which about 853 were considered to be caused by radiation. The weapons used on the two cities were very different. The one used on Nagasaki was of a type that would be expected to emit gamma rays with few neutrons and had been previously tested, so dosimetry is based partly on measurements. The weapon used at Hiroshima was of a type never tested before or since, so that dose estimates are based largely on computer simulations. The radiation from this weapon was a mixture of neutrons and γ-rays. The dosimetry relating to the atomic bombs has been revised several times over the years, leading to changes in the cancer risk estimates. The most recent estimates were published in the Biologic Effects of Ionizing Radiation (BEIR) VII report in 2006 and will be discussed later in this chapter.
In Britain, from 1935 through 1944, some 14,000 patients suffering from ankylosing spondylitis were given radiotherapy to various regions of their spine to relieve pain. A small risk of leukemia mortality has been reported in these patients. Although the spondylitic series provides one of the largest bodies of data on leukemia in humans after exposure to x- or γ-radiation, and the dosimetry is quite good, it is far from ideal because it lacks a proper control, consisting of patients with the same disease who did not receive x-ray therapy but whose treatment was otherwise the same. A possible contribution
of carcinogenic drugs to the tumor incidence also has been suggested.
There is also documentation of an elevated incidence of leukemia in radiologists who joined learned societies before about 1922, before the introduction of radiation safety standards. This will be discussed later in the chapter.
Thyroid cancer has been observed in children who received radiotherapy for what was thought to be an enlarged thymus. The thyroid was included in the treatment field, and both malignant and benign thyroid tumors have been observed. Breast cancer is also elevated in these patients.
Until the 1950s, it was common practice to use x-rays to epilate children suffering from tinea capitis (ringworm of the scalp). An increased incidence of thyroid cancer from this practice was first reported by Modan and his colleagues in Israel, who treated more than 20,000 immigrant children from North Africa in whom ringworm of the scalp reached epidemic proportions. There was also a significantly increased risk of brain tumors (mostly meningiomas), salivary gland tumors, skin cancer, and leukemia mortality. A comparable group of children in New York for whom x-rays were used for epilation before treatment for tinea capitis show quite different results. There were only two malignant thyroid tumors in addition to some benign tumors. There is, however, an incidence of skin cancer around the face and scalp in those areas also subject to sunlight. The skin tumors arose only in white children and there were no tumors in black children in the New York series.
Patients with tuberculosis, who were fluoroscoped many times during artificial pneumothorax, have shown an elevated incidence of breast cancer. This was first reported in Nova Scotia, but the report was confirmed by a similar study in New England. The doses these patients received are uncertain but must have been about 0.8 to 0.9 Gy, because some of the women developed skin changes in the chest wall on the side frequently fluoroscoped. Patients who received radiotherapy for postpartum mastitis were also shown to have an excess incidence of breast cancer.
▪ THE LATENT PERIOD
The time interval between irradiation and the appearance of a malignancy is known as the latent period.
Leukemia has the shortest latent period. Excess cases began to appear in the survivors of Hiroshima and Nagasaki a few years after irradiation and reached a peak in 5 to 7 years; most cases occurred in the first 15 years. Solid tumors show a longer latency than the leukemias, on the order of anything from 10 to 60 years or more. For example, an excess incidence of solid tumors is still evident in Japanese survivors exposed to radiation from the atomic bombs in 1945. Indeed, for solid cancers, the excess risk is apparently more like a lifelong elevation of the natural age-specific cancer risk.
As the Japanese data have matured, the concept of a fixed time interval between irradiation and the appearance of the malignancy has been replaced by or combination of “age at exposure” and “time since exposure.” Regardless of the age at the time of exposure, radiation-induced solid tumors tend to be expressed later in life, at the same time as spontaneous tumors of the same type. Breast cancer in women is the most striking example. This suggests that although radiation may initiate the carcinogenic process at a young age, additional steps are required later in life, some of which may well be hormone dependent.
▪ ASSESSING THE RISK
To use the available human data to estimate risks as a function of dose, it is necessary to fit the data to a model. Several reasons for this are as follows:
Data obtained at relatively high doses must be extrapolated to the low doses of public health concern.
No large human population exposed to radiation has yet been studied for its full life span, and so estimates must be projected into the future. For example, in the year 2000, about half of the Japanese survivors irradiated in 1945 were still alive.
The best data pertain to the Japanese irradiated by the atomic bombs and risk estimates based on this must be transferred to other populations that have quite different characteristics, including their natural cancer incidence.
There are two types of models that are conceptually quite different: the absolute risk model and the relative risk model. The absolute risk model assumes that radiation induces a “crop” of cancers over and above the natural incidence unrelated to it. The relative risk model assumes that the effect of radiation is to increase the natural incidence at all ages subsequent to exposure by a given factor. Because the natural or spontaneous cancer incidence rises significantly in old age, the relative risk model predicts a large number of radiation-induced cancers in old age.
The model favored by recent BEIR committees, for the assessment of the cancer risks from the Japanese atomic bomb survivors is the time-dependent relative risk model. The excess incidence of cancer was assumed to be a function of dose, the square of the dose, age at exposure, and time since exposure. For some tumors, gender must be added as a variable—for example, in the case of breast cancer.
▪ COMMITTEES CONCERNED WITH RISK ESTIMATES AND RADIATION PROTECTION
There are two series of reports that analyze available data and come up with risk estimates for radiation-induced cancer. The first is the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) reports. This committee reports to the General Assembly at regular intervals; the most recent report appeared in 2000. The second is the committee of the U.S. National Academy of Sciences known as the Biologic Effects of Ionizing Radiation (BEIR). Reports appear periodically, the most recent comprehensive report (BEIR VII) appearing in 2006. To a large extent, these are “scholarly” committees, inasmuch as they are under no compulsion to draw conclusions if data are not available.
On the other hand, there are committees involved with radiation protection that cannot afford to be scholarly because they must make recommendations whether or not adequate data are available. First, there is the International Commission on Radiological Protection (ICRP). This commission was originally set up and funded by the first International Congress of Radiology. Over the years, the funding base of this commission has broadened, and it has assumed the role of an independent, self-propagating committee. At a national level in the United States, there is the National Council on Radiological Protection and Measurements (NCRP). This is an independent body chartered by Congress and is funded from industry, government grants, and professional societies. The NCRP formulates policies for radiation protection in the United States often, but not always, following the lead of the ICRP. The recommendations of the NCRP carry no weight in law but are usually adopted eventually and enforced by the regulatory agencies in the United States, although there can often be a long lag period. (See Chapter 17 on radiation protection for more regarding these committees.)
▪ RADIATION-INDUCED CANCER IN HUMAN POPULATIONS
Under appropriate conditions, a malignancy can be induced in essentially all tissues of the body. Some of the most common are discussed below.
Leukemia
The incidence of chronic lymphocytic leukemia does not appear to be affected by radiation. Acute and chronic myeloid leukemia are the types chiefly responsible for the excess incidence observed in irradiated adults. Susceptibility to acute lymphatic or stem cell leukemia seems to be highest in childhood and to decrease sharply during maturation.
Two principal population groups provide data to determine risk estimates:
Survivors of the atomic bomb attacks on Hiroshima and Nagasaki
Patients treated for ankylosing spondylitis
Leukemia was the first malignancy to be linked with radiation exposure in the A-bomb survivors and has the highest relative risk of any malignancy. Leukemia risks increased with dose up to about 3 Sv, with evidence of upward curvature; that is, a linear-quadratic function of dose fits the data significantly better than a linear function. Because of this curvature, the risk per unit of dose at 1 Sv is about three times greater than at 0.1 Sv.
Thyroid Cancer
The thyroid gland is an organ of high sensitivity for radiation carcinogenesis, at least in children; in adults, radiation is much less efficient in inducing thyroid cancer. The malignant tumors that have been produced, however, consistently have been of a histologically well-differentiated type, which develops slowly and often can be removed completely by surgery or treated successfully with radioactive iodine if metastasized; consequently, these tumors show a low mortality rate. It is estimated that about 5% of those with radiation-induced thyroid cancer die as a result.
The following are the principal population groups available for deriving risk estimates for thyroid cancer:
Survivors of the atomic bomb attacks on Hiroshima and Nagasaki.
Residents of the Marshall Islands exposed to external radiation and ingested iodine-131 from fallout after the 1954 testing of a thermonuclear device, in whom there was a high incidence of nodule formation and some thyroid cancer (benign as well as malignant tumors).
Individuals who ingested radioactive iodine as a result of the Chernobyl accident (this experience shows how very sensitive children are and that adults are relatively resistant).
Children treated with x-rays for an enlarged thymus.
Children treated for diseases of the tonsils and nasopharynx.
Children epilated with x-rays for the treatment of tinea capitis.
Children treated for cancer.
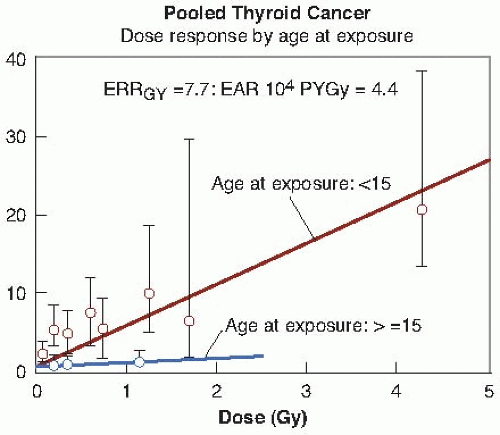
Figure 10.3 shows the relative risk for thyroid cancer after exposure to external radiation, taken from a pooled analysis of seven different studies, which dramatically illustrates the importance of age at exposure.
Breast Cancer
Breast cancer may be induced with relatively high frequency by radiation. The cancer is of the type arising initially from duct cells but is commonly found to infiltrate breast tissue.
There are three principal exposed populations from which the risk of breast cancer incidence may be derived:
Japanese female survivors of the atomic bomb attacks on Hiroshima and Nagasaki.
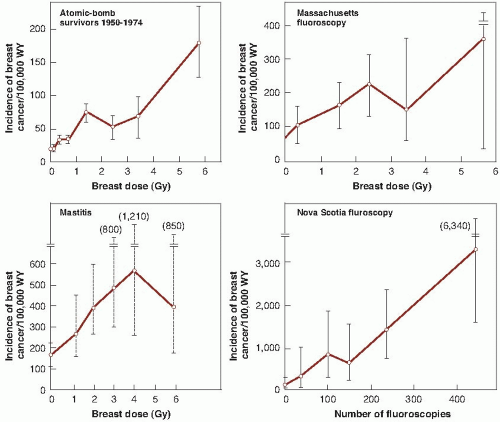
Female patients in a Nova Scotia sanatorium subjected to multiple fluoroscopies during artificial pneumothorax for pulmonary tuberculosis. There is doubt about the dosimetry, but the dose to breast tissue per fluoroscopy is estimated to have been 0.04 to 0.2 Gy. The number of examinations commonly exceeded 100, and in some instances, women received more than 500 fluoroscopies; three patients, in fact, developed radiation dermatitis. This group of exposed women probably constitutes the most convincing evidence of the production of cancer by fractionated x-rays used for diagnosis. This Canadian study also showed the importance of age at the time of exposure. The study was later confirmed by the follow-up of patients discharged from two tuberculosis sanatoria in Massachusetts. These patients were examined fluoroscopically at an average of 102 times over a period of years and, subsequently, were
found to be 80% more likely to develop breast cancer than a comparable unexposed population.
Females treated for postpartum mastitis and other benign conditions. Patients typically received 1 to 6 Gy and showed an excess incidence of breast cancer compared with the general female population of New York State. A legitimate objection to the use of these data for risk estimates is the uncertainty of whether postpartum mastitis predisposes to breast cancer.
The data for excess incidence of breast cancer in these populations are shown in Figure 10.4. Several interesting points are immediately apparent. First, the data from the New York series of postpartum mastitis patients are so poor that they do not give any clue about the shape of the dose- response relationship. Second, there is a marked difference in the natural incidence of breast cancer in Japanese women in whom it is low, compared with American and Canadian women in whom it is high; nevertheless, in all cases, incidence rises with radiation dose. Third, the data for breast cancer are reasonably well fitted by a straight line.
Lung Cancer
Radiation is but one of a long list of carcinogens for lung cancer: Cigarette smoking, asbestos, chromium salts, mustard gas, hematite, and asphalt derivatives have also been implicated.
Radiation risk estimates come from two principal sources:
Radiation risk estimates come from two principal sources:
Individuals exposed to external sources of radiation, including the Japanese survivors and those with ankylosing spondylitis. An excess was found even when smoking was taken into account.
Underground miners exposed to radon in the mine atmosphere. The naturally occurring deposits of radioactive materials in the rocks of the earth decay through a long series of steps until they reach a stable isotope of lead. One of these steps involves radon, which, unlike the other elements in the decay series, is a gas. In the closed environment of a mine, workers inhale radon gas and some radon atoms decay to the next solid member of the radioactive series, which consequently is deposited on the bronchial epithelium. Subsequent steps in the radioactive decay series take place in the lungs, causing intense α-irradiation of localized surrounding tissue.
There is a clear excess of lung cancer among workers in the uranium mines of the Colorado plateau in the United States, the uranium mines in Czechoslovakia, the nonuranium mines in Sweden, and the fluorspar mines in Newfoundland. It remains difficult to separate adequately the contributory effects of radon and cigarette smoking in causing the cancers, because there are too few nonsmoking miners to form an adequate control group. In addition, the average duration of exposure usually spans from 15 to 20 years, during which standards of safety and ventilation have changed substantially. In any case, it is no easy matter to estimate the dose to the critical cells in the basal layer of the epithelium of the lung from knowledge of the radon concentration in the air that is breathed. There is also some evidence, summarized in the BEIR VI report, of an excess of lung cancer from domestic radon exposure. It is estimated that 10% of the 150,000 lung cancer deaths annually in the United States are caused by radon.
Bone Cancer
There is some evidence of bone cancer induced by external x-irradiation in children epilated for the treatment of tinea capitis and in patients treated for ankylosing spondylitis. The numbers are small and the risk estimates poor. The largest body of data comes from two populations, each of which ingested isotopes of radium that emit high linear energy transfer (LET) α-particles and that follow the metabolic pathways of calcium in the body to become deposited in the bone. The populations include the following:
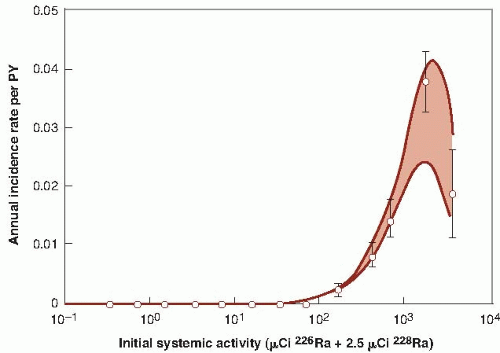
Young persons, mostly women, employed as dial painters, who ingested radium as a result of licking their brushes into a sharp point for application of luminous paint to watches and clocks. In this group, there have been bone sarcomas and carcinomas of epithelial cells lining the paranasal sinuses and nasopharynx. None of these tumors occurred at doses below 5 Gy; above this level, the incidence rose sharply, particularly the sarcomas. The radium in these paints consisted of the isotopes radium-226 and radium-228, with half-lives of about 1,600 years and 6 years, respectively.
Patients given injections of radium-224 for the treatment of tuberculosis or ankylosing spondylitis.
There are three points that need to be emphasized. First, the dose is made up of α-particles, which have a short range and deposit their energy close to the site at which the isotope is deposited; α-particles are also more effective than x-rays by a factor of about 20. Second, osteosarcomas arise predominantly from endosteal cells, and the relevant dose for estimating the risk of sarcoma is the dose to these cells, which lie at a distance of up to 10 µm from the bone surface, rather than the mean dose throughout the bone. Radium-224 has a short half-life (3.6 days), and its radiation therefore is largely delivered while it is still present on the bone surface. This contrasts sharply with radium-226 and radium-228, which have long half-lives and, consequently, become distributed throughout the bone during their periods of radioactive decay. The dose to endosteal cells from radium-224 is about nine times larger than the dose averaged throughout bone, whereas it is about two-thirds of the mean bone value from radium-226. Consequently, it is difficult to compare data from the two groups of people who were exposed to these very different
isotopes of radium. Third, age at the time of exposure is an important factor in the development of bone cancer. For young persons, and possibly for those exposed in utero, the rapid deposition of bone-seeking radioisotopes during active bone growth might confer a higher risk of cancer than in adults. There is, in general, poor agreement among the risk estimates derived from the various groups of persons showing an excess of bone cancer, so that risk estimates must be very crude. Figure 10.5 shows the incidence of bone sarcoma in female dial painters as a function of activity of radium ingested. These data imply that a linear extrapolation from high to low doses would overestimate risks at low doses. It appears that sarcomas are induced only after large doses that are sufficient to cause tissue damage and, therefore, to stimulate cell proliferation.
isotopes of radium. Third, age at the time of exposure is an important factor in the development of bone cancer. For young persons, and possibly for those exposed in utero, the rapid deposition of bone-seeking radioisotopes during active bone growth might confer a higher risk of cancer than in adults. There is, in general, poor agreement among the risk estimates derived from the various groups of persons showing an excess of bone cancer, so that risk estimates must be very crude. Figure 10.5 shows the incidence of bone sarcoma in female dial painters as a function of activity of radium ingested. These data imply that a linear extrapolation from high to low doses would overestimate risks at low doses. It appears that sarcomas are induced only after large doses that are sufficient to cause tissue damage and, therefore, to stimulate cell proliferation.
Skin Cancer
The first neoplasm attributed to x-rays was an epidermoid carcinoma on the hand of a radiologist, which was reported in 1902. In the years that followed, several hundred of such cases arose among physicians, dentists, physicists, and x-ray technicians, in an era in which safety standards were virtually nonexistent. In most cases, the onset of neoplasms followed chronic radiodermatitis and a long latent period. Squamous cell and basal cell carcinomas have been most frequently observed, and occasionally, a sarcoma of the subcutaneous tissues has been seen. Since the evolution of modern safety standards, epidermoid carcinoma has ceased to be an occupational disease of radiation workers.
Radiation-induced skin cancers are diagnosed readily and treated at an early stage of development, and there is a large difference between rates of incidence and mortality. There is a small excess incidence of skin cancer in the children epilated with x-rays for the treatment of tinea capitis.
▪ QUANTITATIVE RISK ESTIMATES FOR RADIATION-INDUCED CANCER
Despite a diverse collection of data for cancer in humans from medical sources, both the BEIR and UNSCEAR reports elect to base their risk estimates almost entirely on the data from the survivors of the atomic bomb attacks on Hiroshima
and Nagasaki. Figure 10.6 summarizes the study groups available from the Radiation Effects Research Foundation (RERF).
and Nagasaki. Figure 10.6 summarizes the study groups available from the Radiation Effects Research Foundation (RERF).
The Life Span Study (LSS), comprising about 120,000 people, allows estimates to be made of the radiation-induced cancer incidence and cancer mortality.
The in utero cohort, comprising about 3,300 people who were exposed to radiation from the bombs while in utero, allows estimates to be made of the incidence of malformations, growth retardation, microcephaly, and mental retardation.
The children of the exposed persons, the socalled F1 generation, allows estimates to be made of heritable effects.