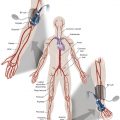
The x-ray equipment available to perform imaged-guided interventions has accelerated in technical capability, allowing increasingly complex procedures to be performed. Consequently, procedures tend to be longer and the dose to the patient higher. The interventionalist is positioned closest to the anatomy being imaged and receives the majority of their exposure from x-ray radiation scattering from the patient. Typically, measures that reduce the dose to the patient also reduce the dose to staff. Circulating staff in the room may also receive high scatter doses, particularly the nurses providing sedation to the patient and communicating with them. The staff’s positioning when carrying out these tasks may mean that they are not protected by any additional in-room lead shields.
Ionizing Radiation Risks
Stochastic Effects
The Oxford English Dictionary defines stochastic as “randomly determined; having a random probability distribution or pattern that may be analyzed statistically but may not be predicted precisely.” The need for radiation protection arises from the detrimental effects, including those that are stochastic or random, that can occur due to exposure to ionizing radiation. Our main knowledge regarding radiation effects has been obtained from studying the survivors of the atomic bombs (known as the Life Span Study [LSS]). However, the doses from these acute exposures were delivered much faster (higher dose rate), were high linear energy transfer radiation, and in a higher dose range (much greater than 100 millisieverts [mSv]) than is typically delivered in a diagnostic or interventional medical imaging procedure. Radiation-induced cancer is one of the main outcomes studied in the LSS. These cancers can appear up to years after the exposure (latent period) and are stochastic in nature. When translating (by extrapolation and scaling to take account of the lower dose rate and dose) the results from the atomic bomb survivors, the dose response for solid cancer incidence follows a linear function with leukemia incidence better described by a linear-quadratic fit.
As interventionalists we are chronically exposed to low-dose radiation. This is a very different phenomenon from the atomic bomb survivor population. This leads to controversy. There are polarized schools of thought about the risk of chronic low-dose radiation. On one end of the spectrum are those who say no exposure is safe. On the extreme far end are people who believe that radiation exposure promotes cell mutation that may result in beneficial evolution of humanity. This phenomenon is called hormesis . Large national studies from developed countries like South Korea report the long-term risks of chronic low-dose radiation in radiographers, radiologists, industrial radiographers who use cobalt sources to x-ray steel in aircraft turbine blades and buildings, and nuclear power station workers. Other international studies pool data on survivors of Chernobyl, employees of the nuclear power industry, and those exposed during nuclear accidents such as Fukushima. These studies include hundreds of thousands of workers with occupational exposure and their long-term follow-up. From these studies the concept of the linear no threshold model has evolved. This essentially conveys that the risk of cancer increases approximately with dose, and risk exists even at the lowest doses. At higher doses where the likelihood of multiple interactions in a cell increases, the shape of the response curve is more likely to be linear-quadratic. For low linear energy transfer radiation and low dose rates there is a good chance of repair between radiation insults.
At the low end of the dose range encountered in medical imaging, the linear no threshold response is the premise used for radiation protection purposes within the clinical environment. However, controversy regarding the actual shape of the dose-response curve in this low-dose region remains. It is most likely that the linear no threshold model is a conservatively safe basis for radiation protection.
It is difficult to show a causal connection with radiation and cancer directly in either patient groups undergoing medical imaging or staff who have been occupationally exposed. Roguin et al. have facilitated a quasi registry of brain cancers in interventionalists (n=31) primarily through self-reporting. Their findings show a greater percentage (22 of 26 cases, 85%) of left-sided malignancy , which is speculated to correlate with the higher levels of radiation typically received on the left side of the head because of the positioning of the interventionalist during a case. Obviously whether these cancers are radiation induced and whether they demonstrate an excess over natural incidence cannot be established with only anecdotal reporting.
A large-scale epidemiology study of US radiologic technologists found no evidence of an excess risk of malignant intracranial tumor due to occupational radiation exposure, including in a subcohort with consistently higher brain doses who assisted with fluoroscopy-guided interventions. However, they did find an excess risk for unknown reasons among those technologists who had ever (compared with those who had never) worked in fluoroscopy-guided procedures. They observe that these results do not necessarily mean that there is no association between radiation and brain tumors, but instead that the small number of cases and the low brain doses (mean cumulative weighted brain dose of 12 milligray [mGy]; range, 0–290 mGy) may limit identification of any causal connection.
Similarly, recent large-scale studies of radiologists and physicians likely to perform image-guided interventions using a control group of psychiatrists found no association between occupational radiation exposure and cancer. Dosimetry was not available for these studies, so dose response was not assessed. However, excess mortality risks were seen in radiologists and physicians who graduated before 1940, seemingly correlating with higher dose exposure in the earlier years.
The other type of stochastic response to radiation exposure is heritable effects, where exposure of the reproductive cells may lead to disease in the children of the person exposed. These types of genetic effects are considered to follow the same linear no threshold dose response for the purpose of radiation protection. Interestingly, there is no direct evidence of heritable effects in human studies, although experiments with animals have shown otherwise. Overall, the International Commission on Radiological Protection (ICRP) recommends a risk coefficient of 5% per sievert of radiation for fatal stochastic effects. At the dose levels relevant to medical imaging this equates to 0.005% per millisievert (mSv) or a risk of fatal radiation-induced cancer of 1 in 20,000 per mSv. It is essential to recognize that this is a population risk rather than specific to an individual.
At a cellular level, radiation exposure can cause direct damage to DNA resulting in double- or single-stranded breaks (lesions) or indirect damage through the production of reactive oxygen species that also lead to DNA damage. Repair begins immediately, with opportunity for full repair or misrepair resulting in chromosomal aberrations. It has been shown that the cellular damage mechanism may be inhibited or modulated and/or repair mechanisms up-regulated by a variety of radioprotective agents . It is thought that reducing damage will ultimately reduce the risk of radiation-induced carcinogenesis. However, this is difficult to prove because it would require long-term follow-up through epidemiologic studies. To date, most research in this area has been achieved through in vitro experimentation, and although some agents are used to reduce radiotherapy side effects, none have been administered outside of research in diagnostic or interventional imaging. Velauthapillai et al. have shown that oral antioxidants taken 1 hour before technetium-99m methylene diphosphonate bone scans can block 90% of DNA breaks seen in the control group. This could lead to patients and healthcare workers being medicated before radiation exposure. This was an entirely in vivo study.
Assessments of cellular damage are usually achieved through quantifying radiation-induced double-stranded breaks through a biomarker, γ-H2AX. Radiation insult leads to activation of a response through protein kinase, predominantly by ataxia-telangiectasia-mutated (ATM), which causes phosphorylation of the histone H2AX producing γ-H2AX. The levels of γ-H2AX (as a damage/repair marker) and phosphorylated ATM (as a response marker) are time dependent after radiation exposure and have become more readily quantifiable through cytometry using fluorescence-activated cell sorting. Despite the difficulty in performing in vivo research in this area, a recent study using blood samples taken from interventionalists before and after performing image-guided endovascular aneurysm repair (EVAR) cases showed that levels of both γ-H2AX and pATM increased significantly. Interestingly, levels of both markers normalized after 24 hours.
In the study of interventionalists performing EVAR procedures, blood samples were also exposed to known doses of radiation in vitro. The results demonstrated a variable individual response to radiation, as measured by γ-H2AX prevalence. This study and others have suggested that individual susceptibility to radiation exists. It is recognized that people with genetic disorders involving an impaired genetic response to DNA repair—for example, ataxia telangiectasia—have higher susceptibility to radiation effects or are considered more radiosensitive. It is worth considering.
One feasible risk-reduction method would be the development of a prescreening procedure for current or future interventionalists for inherited mutations that would predispose individuals to radiation-induced cancer. The first step would be to look at family cancer history and to identify high-risk populations. Some of this is simple population genetics. The Ashkenazi Jewish population, for instance, has a 2% chance of carrying a BRCA germ-line mutation that predisposes to impaired p53-mediated DNA, the consequence of which manifests as increased risk of breast, ovarian, peritoneal, and prostate cancers. Interestingly, concern has been raised regarding the potentially carcinogenic effects of low-dose ionizing radiation from annual or even biannual mammograms in BRCA1 and/or BRCA2 mutation carriers. If we are concerned about biannual low-dose mammographic radiation breast cancer induction, should we not be concerned about the higher doses potentially received by interventionalists who carry BRCA1/2 mutations?
A follow-up step might be to offer potentially at-risk interventionalists and trainees access to genetic screening for germline mutations (likely including mutation types in BRCA1, BRCA2, and MLH1, among others) that increase susceptibility to radiation-induced DNA damage. Certainly, screening for specific mutations is a well-established and increasingly affordable practice in the context of patients with positive family histories of breast, ovarian, and colorectal cancers. The intent of such a screening process would be to provide an objective estimate of the risk an individual might incur by pursuing a career in interventional radiology, and to allow them to make informed decisions on the basis of that knowledge. The choice of what to do in the event that impaired DNA repair ability were identified would be completely up to the individual; perhaps an early trainee would choose to pursue a magnetic resonance imaging or ultrasound intervention-based career, whereas an established interventionalist might heighten their adherence to lead barrier-based risk-reduction methods or reduce the number of high-exposure procedures they perform each year. Of course, the potential ethical ramifications of such screening would have to be considered carefully. For one, the test results would need to be strictly confidential to safeguard against any possible discrimination, however well meaning, that might ensue from their dissemination. Screening would have to be performed strictly in the context of appropriate counselling and informed interpretation. Finally, participation in screening would have to be purely optional, with the choice to know more about their genetic risk profile left up to each individual.
Tissue Reactions
Radiation exposure can also induce a reaction in the tissues or organs exposed. Tissue reactions are similarly known as deterministic effects . These are typically evident on a much shorter time frame than stochastic effects and occur above a threshold and hence are not probabilistic in nature. As doses increase above the threshold the severity of the effect also increases. The most common tissue reactions for patients seen in image-guided interventional procedures are those occurring in the skin. Above a threshold, which is thought to be around 2 Gy, the initial effect will be transient erythema, but as doses increase this can progress to permanent epilation, radiation-induced telangiectasia, dermal atrophy or induration, and, at particularly high doses, necrosis with possible surgical intervention necessary.
Radiation-induced skin injury is well known in radiotherapy. However, it is relatively rare in image-guided procedures. In fact, it is likely that an interventionalist may not observe a skin effect in a patient during their working lifetime when using good practice in an environment with a radiation protection program. There are many reported cases of skin injury despite being infrequent. When an injury occurs, it can be severe and significantly affect quality of life. Some injuries result from patient factors (obesity, previous radiation exposure); others may be considered avoidable (machine malfunction, unintentional direct irradiation of body parts (arms), and lengthy procedures with no real-time dose feedback. In addition, the medications that the patient is taking should be considered. A patient undergoing chemotherapy will have diminished cellular repair ability and may be at higher risk of radiation dermatitis.
Again, the issue of individual sensitivity to radiation exposure is relevant. Knowledge from both radiotherapy and image-guided interventional procedures that produce skin injury shows that predisposing factors for radiation-induced skin damage include smoking, diet, and impaired skin integrity as well as an increase in severity when combined with certain chemotherapy agents even at a period of months postprocedure in so-called radiation recall. It has been suggested that follow-up by a dermatologist would identify more skin injury than follow-up by a radiologist.
Although not well recognized, radiation-induced circulatory effects (cardiovascular and cerebrovascular, such as heart attack and stroke) are noncancer outcomes that have also been observed in the LSS and radiotherapy patients. These are believed to be due to elevated circulating reactive oxygen species resulting in a chronic low-grade inflammatory state. The ICRP first included circulatory disease as a radiation outcome in 2012, with a threshold dose of 0.5 Gy for acute, fractionated, and chronic exposures. It is acknowledged that much uncertainty remains, hence the application of a single threshold regardless of dose rate. These effects, if occurring, are relevant to patient exposure of the heart and brain in complex interventional procedures where doses may exceed these values. The threshold dose is defined as the level at which 1% incidence of the detrimental effect in the exposed population is expected. For circulatory disease, this is a small increase over the natural incidence of 30% to 50% and the radiation-induced effect is expected to occur after a latency of at least 10 years.
For interventional staff, it is becoming increasingly understood that opacification of the lens of the eye, or cataracts, is a potential risk from radiation due to occupational exposure. As vision impairment worsens, cataract surgery can be required. In revising the scientific literature, the ICRP in recent years has lowered the theoretical threshold for radiation-induced cataract induction to 0.5 Gy for acute and protracted exposures. This is indicative of the higher radiosensitivity of the lens compared with other tissues. In fact, it is not clear whether cataract formation is potentially a stochastic effect because there is considerable uncertainty regarding the dose-response mechanism in the lens of the eye and if a threshold is indeed applicable.
Several factors appear to increase the risk of radiation-induced cataract, including increasing age, gender (female), marital status (single), socioeconomic status (low), smoking history, and body mass index, among others. However, these also tend to be predisposing factors for natural incidence, making it difficult to isolate the radiation causal relationship.
Fetal Effects
Radiation exposure in utero is known to cause threshold-based effects such as prenatal death, malformation, reduction in IQ, and a stochastic risk related to childhood leukemia and cancer. Understandably this causes anxiety both for the patient and for the interventionalist who must decide whether to perform the procedure, but also for pregnant interventionalists and other staff who may worry about the exposure of their unborn child. The natural instinct is to avoid radiation exposure of an unborn baby given that some risks do exist. However, there is potentially a lack of understanding or knowledge regarding fetal irradiation because often the medical exposure of a pregnant patient will be readily justified. It is likely that an interventionalist may need to perform a procedure on a pregnant patient during their career.
The thresholds for detrimental effects are generally thought to be above the range 0.1 to 0.2 Gy (100–200 mGy). Conceptus/fetal doses at these levels will only be reached in situations with prolonged direct abdominopelvic patient exposures. Furthermore, incidence of an effect is dependent on the gestational age at exposure and the dose received. Preimplantation (0–2 weeks) exposure is thought to be an “all or nothing” effect resulting in death of the conceptus at doses above 0.1 Gy (or potentially less). This is based on animal data because human conception is typically not detected at this time. Maximum sensitivity for malformation is during major organogenesis (3–8 weeks) and for IQ reduction during brain development (8–15 weeks) with continuing sensitivity in weeks 16 to 25, again both with a threshold around 0.1 Gy, although this may be lower. Cancer risk is believed to be applicable from exposure during major organogenesis (from 3 weeks) to term. The risk coefficient for fatal cancer is, at most, a few times the population as a whole. Termination of pregnancy should not be considered at doses below 100 mGy and even above this threshold consideration needs to be given to the gestational age at exposure and the dose. When counseling patients it can be useful to describe the radiation risk in the context of the natural risk of not having a child with cancer or a birth malformation and the change to the probability as a result of radiation exposure.
Practical Radiation Protection
Protective Equipment
For an interventionalist, the full range of personal protective equipment available and recommended can be burdensome, particularly for the duration that it needs to be worn. However, getting into a routine practice of utilizing protective equipment is considered essential given the increasing occupational doses within the interventional suite and the expanding scientific knowledge of deleterious effects from exposure. Ideally, an interventionalist and staff within the room should wear at least 0.35 mm lead equivalent apron , thyroid collar , and lead glasses with side shields. Skirt and vest options are recommended because these are typically more back-friendly. It is not advisable to wear aprons of the open-back style. This ensures protection irrespective of staff location, especially for scrub and scout nurses and radiographers/technologists who may be circulating in the room. It is important to ensure that the apron is a good fit, both ergonomically and for radiation protection. It should be knee length and well-fitting around the armpits to ensure minimal radiation is able to penetrate the chest, particularly because the arms may be partially raised during a procedure. Thyroid collars are also important because the thyroid is one of the most radiosensitive parts of the body, particularly for younger people (under 20 years of age).
Aprons and collars should be checked annually for lead integrity . Aprons made of lead material typically last 10 years when well cared for (hung appropriately and cleaned). The lead equivalence should be first checked by a medical physicist to ensure that sufficient protection is provided. New batches of aprons should be fluoroscopically screened on delivery to ensure integrity (not patchy or holey) and the claimed lead thickness. It is simple to have some small squares (e.g., approximately 8×8 cm) of lead (0.1 mm thick) and place these next to the apron in multiples of 0.1 mm when being screened. The equivalence of protection when compared with known lead thickness is readily evident. For some lightweight materials the longevity of the aprons appears reduced. There is anecdotal evidence of the protective insert material dropping to the bottom of the outer fabric. There may need to be better attention paid to the care of these aprons and the weight-bearing points when they are hung.
Most facilities are considering the mandatory adoption of lead glasses in interventional suites, or at least of the primary operator, to prevent cataract induction and meet new dose limits to the lens of the eye that have been regulated in some jurisdictions. To be effective, these glasses should be fitted to the individual because face shape can significantly affect how protective the glasses will be. The type of nose pad/bridge of the glasses will affect comfort based on individual preference. Scattered radiation also penetrates from the side, and glasses with side shields are preferred, although this does add to the weight of the glasses. Those people who typically wear glasses to correct eyesight may find it easier to adopt wearing lead glasses because they are used to the feel and weight. However, most people report becoming comfortable with wearing lead glasses over time. A head strap is essential to keep the glasses in place, particularly when looking down. Certain makes of glasses can incorporate prescription lenses. This is usually unavailable in lenses that have a high degree of curvature. A typical complaint when wearing lead glasses is that they fog and reduce vision. This can be addressed by using an antifog spray (similar to those used for diving goggles) and ensuring that the surgical mask makes good contact with the nose to prevent air rising and fogging the glasses. Facilities should ensure that there are some common-use lead glasses available (and overglasses for those staff wearing ordinary prescription glasses). If individual lead glasses are not provided or privately purchased, then consideration should be given to having enough common pairs of lead glasses available to cover the typical number of staff involved in the laboratory(ies) at any one time.
There has been increasing interest in brain caps with lead equivalence to reduce the risk of intracranial malignancy by shielding the head. However, several studies show that the cap is ineffective, particularly because the scattered radiation is incident on the front of the face and the cap is shielding the back of the head. It is more effective to use the ceiling shield . These days most rooms with interventional capabilities should be fitted with a ceiling-mounted shield that is contoured to fit the patient and protect the upper body and head of the interventionalist. These are constructed of a lead equivalent glass or acrylic, allowing visualization of the patient through the shield. The level of protection depends on positioning of the shield and may need to be adjusted during the procedure to be effective. Lead or rubber curtain drapes are also usually available in some form hanging below the patient table to shield the lower body of the interventionalist from the higher levels of scatter at the x-ray beam skin entrance (for an undertable tube configuration). In rooms where these are not already affixed to the table, mobile lead drape shields can be utilized.
Disposable pads made of nonlead materials (e.g., bismuth) can be placed on the patient outside of the primary beam to reduce scatter to the interventionalist. However, there is not widespread adoption of these as a protective measure, partly due to low cost/benefit ratios and because they may not be suited to all procedure types. If the pad moves into the primary field of view then dose to the patient, and hence the operator, is more likely to increase as the x-ray unit automatically adjusts and tries to penetrate the shielding material. There may be some benefit to using these pads to reduce the interventionalist’s lens dose. The upper body is already well protected by a standard lead apron. However, if the ceiling shield is not being used, or lead glasses are not being worn or are still allowing some scatter to reach the operator’s eye, then there may be some advantage to using the pad.
The disposable pads may also lead to a reduction in interventionalist hand dose, depending on positioning. Alternatively lead-protective gloves can be considered. These used to be bulky, stiff, and heavy and consequently limited the user’s dexterity and were rarely worn. However, much thinner, more flexible materials are now available. The gloves are typically disposable, hence, again, the cost/benefit ratio may not justify their regular use. The best protection for the hands is to ensure that they are not in the primary x-ray beam. Not only does this reduce direct exposure, but it also avoids changes to the x-ray parameters that increase dose to penetrate the additional attenuation in the beam.
The recent study demonstrating DNA damage to interventionalists performing EVAR procedures recommends the use of leg shields . It is not common practice to use leg/tibial shields, and the undertable protective drapes provide significant attenuation of scatter reaching the lower extremities of the interventionalist that are not shielded by the standard lead apron, although others have commented on their routine use.
The effectiveness of distance from the source of radiation (the patient anatomy being imaged is the primary source of scatter) should not be underestimated. Reduction of radiation follows the inverse square law with distance, such that doubling distance will quarter the dose. Although not always practical for the interventionalist who, by necessity, must be close to the radiation source, it should be kept in mind for others within the room.
Operational Safeguards
Only procedures that have been justified as having a net benefit for the patient when considering the radiation risks should be undertaken and all appropriate steps taken to obtain informed patient consent. In approving an interventional procedure, within the clinical context, consideration should be given to any previous radiation exposure of the patient, particularly in the last 60 days (dose review), the length of time between repeated procedures (if relevant), and the gender, age, size (e.g., bariatric), and pregnancy status of the patient. When proceeding with a case, it is important to practice the standard clinical “time out” to ensure that it is the correct patient and correct procedure.
There are several comprehensive guidelines that provide useful information on operational requirements for radiation protection. The International Atomic Energy Agency (IAEA) neatly packages these into the 10 pearls for radiation protection of the patient and staff, which are summarized below. It is recommended to download the freely available posters from the IAEA website, which are provided in multiple languages.
Radiation Protection of the Patient (IAEA 10 Pearls) :
- 1.
Maximize distance between the x-ray tube and the patient to the greatest extent possible.
- 2.
Minimize distance between the patient and the image receptor. It is useful to position the table relative to the tube first (point 1) and then move the image receptor as close to the patient as possible.
- 3.
Minimize fluoroscopy time (keep your foot off the pedal!). Keep records of fluoroscopy time and, if available, dose area product/kerma area product for every patient.
- 4.
Use pulsed fluoroscopy with the lowest frame rate possible to obtain images of acceptable quality.
- 5.
Avoid exposing the same area of the skin in different projections (skin sparing). Vary the beam entrance port by rotating the tube around the patient.
- 6.
Larger patients or thicker body parts trigger an increase in entrance skin dose (ESD).
- 7.
Oblique projections also increase ESD. Be aware that increased ESD increases the probability of skin injury.
- 8.
Avoid the use of magnification. Decreasing the field of view by a factor of 2 increases dose rate by a factor of 4.
- 9.
Minimize the number of frames and cine (acquisition) runs to a clinically acceptable level. Avoid using the acquisition mode for fluoroscopy because these dose rates are about 10 to 60 times the normal fluoroscopy dose rate. Documentation should be performed with last image hold whenever possible and not with cine (acquisition) images.
- 10.
Use collimation (rather than magnification) to collimate the x-ray beam to the area of interest. On most modern machines, virtual collimation allows you to simulate the collimation on a static image so that it can be adjusted without the use of radiation.
Radiation Protection of Staff (IAEA 10 Pearls) :
- 1.
Use protective equipment (discussed in detail in the Protective Equipment section).
- 2.
Make good use of the time, distance, shielding principle: minimize time, maximize distance, utilize shielding.
- 3.
Use ceiling-suspended screens, lateral shields, and table curtains.
- 4.
Keep hands outside the primary beam unless totally unavoidable.
- 5.
Position yourself on the side of the transmitted beam (i.e., by the imaging receptor/detector), where radiation levels are much lower after being attenuated by the patient’s body.
- 6.
Keep the x-ray tube under the patient table and not over it. This provides better protection from scattered dose.
- 7.
Use personal dosimetry (see further discussion in the Dosimetry section).
- 8.
Update your knowledge about radiation protection .
- 9.
Address your concerns about radiation protection to radiation protection specialists (e.g., medical physicists, radiation safety/protection officers).
- 10.
Remember: (1) Quality control testing of fluoroscopy equipment enables safe and stable performance (see further discussion in the Radiation Protection Program section ), (2) know your equipment and use the equipment’s features appropriately, and (3) use injector devices.
It is important to monitor the dose to the patient during the procedure. Using a notification system (generally verbal from the radiographer/technologist) during the procedure prompts the interventionalist to consider the dose already delivered to the patient and the additional radiation necessary to complete the procedure. It is unlikely that a procedure will be stopped due to radiation dose alone, because the clinical benefit of a successful procedure almost always exceeds any detriment to the patient due to radiation. Further, ceasing a procedure partway through may mean that exposure was incurred without corresponding benefit.
Additional dose management procedures may be required postprocedure when the radiation dose to the patient has been significant. Although advice/regulation on this varies, the trigger for additional information could be a skin dose exceeding 5 to 6 Gy. The interventionalist should provide discharge information to both the patient and referrer, and follow-up (in person or by telephone) of the patient 10 to 14 days later is viewed as good practice, particularly if an effect is expected.
Dosimetry
Radiation dose limits apply to occupational exposure, but do not apply to the medical exposure of patients. The dose limits recommended by the ICRP for people working with radiation are an effective dose of 20 mSv per year over 5 years, not to exceed 50 mSv in any one year. Variations on this are implemented in different jurisdictions, so it is best to check the regulatory requirements for your local area.
The conceptus/fetus should be afforded the same level of protection as a member of the public with a dose limit of 1 mSv when the person who works with radiation has declared the pregnancy to the employer. Again, local requirements should be referred to. In most cases, a pregnant staff member can seek a confidential consultation with the radiation safety officer (or equivalent) to review dose history to determine whether any work practice changes are required. More frequent monitoring of radiation dose is usually implemented in order to check more regularly against the dose limit. This consultation also provides an opportunity to discuss any concerns regarding the risks of radiation exposure while pregnant. It is not necessary to use additional lead aprons (and is most likely counterproductive because of the physical weight). Some facilities have a maternity apron available, which may be more comfortable, particularly in the latter stages of pregnancy.
The equivalent dose limit recommended by the ICRP to the lens of the eye is now 20 mSv/year, averaged over defined periods of 5 years, with no annual dose in a single year exceeding 50 mSv (previously this was 150 mSv/year). The annual limit for the hands and the feet is 500 mSv.
There are several commercial dosimetry providers who use a range of dosimeters to measure the dose to an individual. Different countries/jurisdictions vary in requirements on who must wear a personal dosimeter, but also where and how many are to be worn. Because of the level of potential radiation exposure for interventionalists, you should always wear, at a minimum, one personal dosimeter on the inside of the protective apron at chest level. It is preferred that two dosimeters be worn, with a second dosimeter on the outside of the apron at neck level. This dosimeter provides a reasonable indication of the unshielded skin dose and the dose to the lens of the eye (a medical physicist should perform this calculation). However, eye dosimeters are now available that can be worn at eye level (clipped to glasses or cap), which may provide a better estimate of exposure, particularly if the radiation dose behind lead glasses is to be assessed. Note that wearing a single dosimeter underneath the lead apron does not provide an estimate of lens dose.
Finger-ring dosimeters can be worn for procedures where the hands may be close to the primary x-ray beam. Alternatively, short periods of trial monitoring could be undertaken to ascertain typical finger doses. Real-time dosimetry systems are useful. Some of these provide visual feedback, which can assist in proactively reducing exposure.
The dose to the patient is usually displayed on modern interventional equipment in terms of the air kerma (AK) and the dose area product (DAP), which might also be called the kerma area product (KAP). The DAP/KAP is simply the AK at a specific distance from the x-ray tube multiplied by the area of the x-ray beam at the same distance. Given the inverse square law relationship for dose and distance and the rate at which the area of the beam changes with distance, this means that DAP/KAP is constant at any distance from the x-ray tube. It provides a useful indicator of the stochastic risk to the patient. It is determined in air so it does not include the backscatter from the patient, which is relevant when considering the dose to the patient. The additional dose due to backscatter can be up to 50% (typically around 30%), depending on the beam quality (energy and filtration of the x-ray beam) and the beam area. Depending on how the DAP meter is calibrated, it may or may not include table attenuation (most likely not).
On the other hand, AK depends on the distance from the x-ray tube and is expressed at the interventional reference point (IRP). The IRP has different definitions, but for fixed interventional equipment is most commonly 15 cm toward the x-ray tube from the isocenter. This is meant to coincide with the position of the patient’s skin entrance (remember this is most likely on the patient’s back because the x-ray tube should be positioned under the table). However, depending on the setup and the size of the patient, this may be an underestimate if they are closer to the x-ray tube or may be an overestimate if they are further away. The IRP can be determined from the manual for the x-ray equipment or by a medical physicist. The AK can be used by a medical physicist to calculate the peak skin dose.
Peak skin dose is the highest radiation dose at any portion of a patient’s skin during a procedure. It includes contributions from both the primary x-ray beam and from scattered radiation (including backscatter from the patient). It includes all radiation exposure from both fluoroscopy screening and acquisition runs. The peak skin dose is specific to an individual patient and is the quantity used to assess detrimental skin effects. Typically, it is not measured directly or displayed during a procedure, although some manufacturers are starting to offer optional software that does provide peak skin dose information.
Fluoroscopy time is a measure of time not dose. It correlates poorly with the other dose metrics and with the actual skin dose. It should not be used to monitor patient exposure during interventional procedures but may be useful to record to provide information on complexity of case due to length.
Because of the variation in length and complexity of interventional cases, patient doses vary. To approximate effective dose, the DAP/KAP can be multiplied by a conversion coefficient. These coefficients can vary considerably because they depend on the x-ray equipment, x-ray beam quality, size of the patient, and area of the body being exposed. The IAEA provides a collation of these coefficients, which include 0.26 mSv/Gycm 2 for percutaneous transluminal angioplasty (PTA), most embolizations, and transjugular intrahepatic portosystemic shunt (TIPS) procedures and 0.12 mSv/Gycm 2 for pulmonary angiography with filter and bronchial artery embolization. Table 5.1 shows how to convert the displayed DAP/KAP quantity to gray per square centimeter if necessary.