Radio-Theranostics
Carina Mari Aparici
Farshad Moradi
LEARNING OBJECTIVES
1. Define radio-theranostics and how it can contribute to precision medicine.
2. Provide several examples of radio-theranostics in various clinical settings.
3. Describe advantages and limitations of radio-theranostics.
INTRODUCTION
The term theranostics or theragnostics is a portmanteau word derived from the Greek meaning therapy (therapo) and diagnosis/knowledge (gnosis) (1).
Emerging as a specific, safe, and efficient molecular-targeted discipline, theranostics focuses on patient-centered care. It provides a transition from conventional medicine to personalized medicine at the molecular diagnostic and therapeutic level. In the theranostics approach, the first phase is diagnostic, whereas a molecular probe is introduced in the body to obtain high-quality images of a disease (usually malignancies) based on the targeting of a specific biomarker. This phase is followed by the delivery of personalized therapy to the patient, based on the introduction in the body of the same molecular probe (therefore identical biodistribution) although this time with therapeutic properties. The ability to image the molecular and biological pathways of specific diseases/malignancies in the body with these paired probes allows the development of a more selective diagnostic and therapeutic plan tailored to every individual’s disease.
The term theranostics started to appear sporadically in the medical literature at the end of the last century and has steadily increased in frequency of usage (2). Although there are theoretically several theranostic platforms, such as radio-theranostics (the use of radioactive material in the theranostic domain), nanotheranostics, optotheranostics, and magnetotheranostics; radio-theranostics (also known as theranostics by itself), is the only one that is used in the everyday clinical arena. Its unique approach of using targeted-probes already known to the body, in less than microgram levels, labeled with diagnostic or therapeutic natural radiation-emitters of high or low penetration, allows to diagnose or produce a very efficient treatment by trying to target each cell individually, with as low toxicity as possible to healthy (non-targeted) tissues. Thus, theranostics is a holistic evolution from trial and error medicine to informative, predictive, and personalized medicine. In that sense, it achieves the goal of precision medicine, by tailoring and optimizing therapies based on specific characteristics and biology of an individual patient’s disease at the molecular level.
Radio-theranostic pairs
The “concept” of theranostics is not novel to Nuclear Medicine and Molecular Imaging, since radio-theranostics has been an integral part of it since its inception. The backbone of radiotheranostics is the use of a “pair” of chemically and structurally identical or nearly identical radiopharmaceuticals labeled with different isotopes, one for molecular diagnostic imaging and other for molecular targeted radionuclide therapy (TRT) of the same disease. A general approach is to use different radioisotopes of the same periodic element (e.g., I124 for diagnosis and I131 for therapeutics), or the same molecular probe, and label it with different radionuclides based on their diagnostic versus therapeutic properties.
From a puristic point of view, radiolabeling can somehow affect reaction rates and uptake properties of biochemicals compounds; however, as long as the relevant reaction rates (e.g., binding affinity to the receptor) remain fairly similar (e.g., 68Ga-DOTATATE and 177Lu-DOTATATE), it follows the theranostic principle. Theoretically, as the degree of similarity between diagnostic and therapeutic chemicals decreases (even with change in domains that do not directly participate in the reactions), their potential to be used in theranostic applications can be affected. Therefore, the “ideal” theranostic pair would use different isotopes of the same element that are optimal for imaging and therapy (e.g., 86Y and 90Y), respectively. In this case, there is virtually no difference in chemical properties for different isotopes of the same element (excluding hydrogen/deuterium). These “ideal” theranostic pairs have identical reaction rates in the body. The only difference in physical and physiological effects will be due to differences in physical half-life and decay limiting the time course of pharmacokinetics of the radiopharmaceutical and its metabolites.
The quality of the emitting radiation of the radionuclides involved in the theranostic pair is key in their selection for diagnosis or therapy. Each element of the pair should have the radioemission properties that make it more ideal for a diagnostic or therapeutic purpose of the same molecular target. Gamma rays are useful for imaging (diagnosis) since they have very low tissue
absorption and energy transfer, and very long path lengths, resulting in lower radiation to the target and increased but low-level energy radiation to surrounding tissue (emitted for example by 123I). Beta particles on the other hand (emitted for example by 131I, 177Lu, and 90Y) have higher energy transfer to the target (0.2-2 keV/µm) at path lengths that can be up to 11 mm (Table 16.1). Alpha particles (emitted for example by 223Ra) have very high energy transfer (80 keV/µm) and very short path lengths (<100 µm), which are usually the diameter of a few cells. Beta emitters have been proposed for large-/mid-size metastases for the reasons discussed earlier, whereas alpha emitters are considered more advantageous in non-solid tumors and micrometastases. Auger electron emitters (emitted for example by 111In) can be used for therapy as well, although Auger electrons are generally less effective due to very short path length (2-500 nm) and low energy deposition (4-26 keV/mm), and the radionuclide usually needs to be intra-cellular to be effective. Having said all that, rarely a radionuclide has a single type of emission. Most of them have a combination of two or more types of emissions with different abundancies. This property actually makes some of these radionuclides amenable, although not ideal, for both diagnostic and therapeutic purposes. For instance, some beta emitters used for therapy also emit gamma photons and can be used to confirm the molecular targeting after therapy or even dosimetry (e.g., 131I and 177Lu).
absorption and energy transfer, and very long path lengths, resulting in lower radiation to the target and increased but low-level energy radiation to surrounding tissue (emitted for example by 123I). Beta particles on the other hand (emitted for example by 131I, 177Lu, and 90Y) have higher energy transfer to the target (0.2-2 keV/µm) at path lengths that can be up to 11 mm (Table 16.1). Alpha particles (emitted for example by 223Ra) have very high energy transfer (80 keV/µm) and very short path lengths (<100 µm), which are usually the diameter of a few cells. Beta emitters have been proposed for large-/mid-size metastases for the reasons discussed earlier, whereas alpha emitters are considered more advantageous in non-solid tumors and micrometastases. Auger electron emitters (emitted for example by 111In) can be used for therapy as well, although Auger electrons are generally less effective due to very short path length (2-500 nm) and low energy deposition (4-26 keV/mm), and the radionuclide usually needs to be intra-cellular to be effective. Having said all that, rarely a radionuclide has a single type of emission. Most of them have a combination of two or more types of emissions with different abundancies. This property actually makes some of these radionuclides amenable, although not ideal, for both diagnostic and therapeutic purposes. For instance, some beta emitters used for therapy also emit gamma photons and can be used to confirm the molecular targeting after therapy or even dosimetry (e.g., 131I and 177Lu).
Table 16.1 RADIONUCLIDES OF POSSIBLE USE IN THERAPEUTIC THERANOSTICS | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Targeted radionuclide therapy—the “thera” component of the pair
Targeted radionuclide therapy (TRT) is currently a growing treatment option for malignancies. High-affinity molecular probes that carry the radionuclide with therapeutic properties to specific tumor cells based on targeted biomarkers can minimize accumulation of radionuclide in non-target tissues, and therefore significantly reduce side effects. The ideal carrier should have high affinity and specificity for the biomarker expressed by these targeted cells. Commonly used carriers in TRT may include analogs, agonists, or antagonists to selective transporters (e.g., 131I-MIBG acting as a norepinephrine analogue), to selective peptide receptors (e.g., somatostatin receptors type 2 [SSTR2] in PRRT [peptide receptor radiation therapy] with 177Lu-DOTATATE), ligands of membrane proteins/enzymes (e.g., 177Lu-PSMA) or monoclonal antibodies (e.g., radioimmunotherapy with 90Y-ibritumomab tiuxetan).
Internalization and trapping of the radionuclide inside the cell can potentiate the effect of the therapy although is not a requirement. Radiolabeled ligands and small peptides have significant pharmacokinetics advantages to larger molecules such as antibodies or antibody fragments due to faster uptake, more rapid clearance from blood pool activity (particularly resulting in lower radiation to bone marrow), and better penetration in tumors with reduced vascularity, therefore increasing effectiveness and reducing radiation to benign tissues and possible side effect.
The therapeutic effect of ionizing radiation is due to irreversible DNA strand breaks and free radicals. A double-strand break often results in failure to repair DNA and non-viable cells die through induced programmed cell death (apoptosis) (3). Alpha-particles cause irreversible DNA damage if two to three tracks transit the cross-section of the DNA structure, compared to 100 to 1000 tracks for beta-particles (4). Beta-emitting radionuclides are considered to have lower efficacy for tumor micro-deposits than alpha-emitting radionuclides due to its lower linear energy transfer. However, for larger lesions, the “cross-fire” effect between neighboring cells significantly increases the probability of ionization and DNA damage. The “cross-fire” effect is very effective in tumors that have mixed cellularity, some of which no longer expresses the desired molecular target. However, as long as these cells can be within the range of the emitting radiation of the targeted cells, they will also be treated. In addition, radiation and disruption of tumor stroma further contribute to the therapeutic effect. In certain circumstances, long-range penetration is crucial as penetrating radiation can reach cells within the tumor that are difficult to access by the radionuclide, for example, in cases of poorly vascularized tumoral cells. The “cross-fire” effect however is also responsible for causing collateral damage to adjacent benign cells within the range of ionizing radiation from targeted cells, which therefore may cause clinical toxicity and has to be accounted for. The “cross-fire” effect obviously increases with the range of the emitting therapeutic radiation (90Y >> 131I > 177Lu >> 223Ra).
Pseudo-radio-theranostic pairs
Generally, as the degree of similarity between diagnostic and therapeutic chemicals decreases (even with change in domains that do not directly participate in the reactions), their potential to be used in theranostic applications can be affected. In contrast, different biomarkers that target closely linked molecular mechanisms are sometimes very useful as diagnostic-therapeutic pairs. An example is 99mTc-methylene diphosphonate (a bisphosphonate) and 223Ra-dichloride (a calcium analogue). Both have high uptake in areas of bone remodeling and bind to new bone formation, and therefore can be used for diagnosis and treatment of osteoblastic osseous metastatic lesions, respectively. Although the
“strict” definition of radio-theranostics does not encompass these compounds, they definitely seem to work “functionally” as a theranostic tandem or what could be called a “pseudo-theranostic” pair.
“strict” definition of radio-theranostics does not encompass these compounds, they definitely seem to work “functionally” as a theranostic tandem or what could be called a “pseudo-theranostic” pair.
Tc99m-biphosphonate-radium223—bone-seeking pseudo-theranostic pairs
There is a long history of radionuclides being used for imaging and treatment of osteoblastic bone lesions and their pain. The skeleton is a common site of metastasis for certain cancers such as breast and prostate carcinoma, and in most malignancies, they result in increased osseous turnover at the site of the lesion (osteoblastic metastases). This property enables radiopharmaceuticals such as Tc99m-biphosphonates, 18F-NaF, or calcium analogues that get incorporated into the newly formed bone matrix to achieve high uptake in skeletal metastases. Tc99m-biphosphonates, such as Tc99m-MDP, are still the most commonly used radiopharmaceuticals for bone scintigraphy despite the diagnostic superiority of 18F-NaF. They have high sensitivity and specificity compared to conventional imaging for detection and characterization of malignant osteoblastic involvement.
223Ra-dichloride (Xofigo®) is currently the most commonly used radionuclide for bone-targeted therapies. Xofigo® is the first alpha-emitter approved in 2013 by the food and drug administration (FDA) for clinical use. Although 223Ra-dichloride has been approved for treatment of symptomatic bone metastases in castration-resistant prostate cancer whereas, it can also potentially provide a therapeutic alternative in osteoblastic bone metastases of any other malignancy. As a calcium analogue, radium binds to newly formed hydroxyapatite crystals in areas of increased osseous turnover without directly targeting cancer cells but placing itself intimately adjacent to them. Due to the very short path length of the alpha particle emission, its therapeutic effect is primarily due to the disruption of tumor-stroma interaction rather than radiation to malignant cells (5). Consequently, tumor markers such as prostate specific antigen (PSA) are not reliable for response assessment and may lag treatment effects such as reduced bone pain (typically seen after 2 weeks) or decreased alkaline phosphatase. Although flare phenomenon (increased uptake in skeletal lesions on metabolic bone scanning) can initially be seen on imaging, true progression of bone involvement during 223Ra-dichloride treatment is relatively rare.
99mTc-biphosphonates or 18F-NaF can also be paired with therapeutic bone-seeking agents that emit beta radiation. Although internal targeted radiation therapies for osseous lesions using beta-emitting radionuclides strontium-89 (89Sr) and samarium-153 (153Sm-EDTMP) can improve pain in most patients (6,7,8), the effect on survival is limited. Palliation occurs within 4 to 28 days after administration of 89Sr and typically lasts for 3 to 6 months. 223Ra-dichloride administration has a dose-dependent effect in reducing pain, with more than 50% of patients experiencing relief or decreased need for pain medication after intravenous injection of 55 kBq/kg (9).
In a randomized double-blinded multinational phase III clinical trial (ALSYMPCA), patients treated with six injections of 223Ra-dichloride at 4-week intervals had improved survival (14.9 months median overall survival vs. 11.3 months in placebo group), and increased time to symptomatic skeletal events (10). 223Ra-dichloride was safe and effective when used before or after chemotherapy with docetaxel. Concurrent 223Ra-dichloride therapy and chemotherapy are however currently not recommended. Although 223Ra can generally be given safely with abiraterone or enzalutamide (11), adding 223Ra therapy to combination of abiraterone and prednisone does not improve skeletal symptoms and has been associated with increased incidence of pathologic fractures and mortality [ERA-223 trial: 806 patients, increased incidence of fracture 28.6% vs. 11.4%]. 223Ra-dichloride, however, can be considered and appears to be safe in patients who have adequate marrow function after chemotherapy. Recently published results from a small clinical trial suggested that patients who had been previously treated with 223Ra-dichloride and progressed afterwards can be retreated (12). In a 2-year follow-up after retreatment, no serious drug-related adverse events were observed.
The short path length of the emitted alpha particles minimizes hematopoietic marrow toxicity compared to beta-emitting radionuclides (13,14). Although hematopoietic toxicity, gastrointestinal adverse reactions, and peripheral edema were the most common side effects compared to placebo group in the 223Ra-dichloride phase III clinical trial, 223Ra-dichloride therapy is safe and very well tolerated. Severe (grades 3-4) hematologic abnormalities can nonetheless occur in some cases (lymphocytopenia: 20%, anemia: 6%, thrombocytopenia: 3%), and blood count tests are therefore mandatory in the follow-up care of these patients.
Selective internal radiation therapy and pairing
Molecular targeted internal radiation can use biochemical and biological mechanisms to target specific molecules in the lesions to be treated following oral or parenteral administration of a radiolabeled target-specific molecular probe. However, radiolabeled molecular probes can also be delivered via vehicles that can be mechanically placed in the lesion and have an effect in the malignant cells due to proximity (such as brachytherapy, intra-arterial radioembolization, or intra-cavity radionuclide therapy).
Intra-arterial radioembolization of liver lesions has become a common practice nowadays. This practice is based on the unique dual blood supply of the liver and the radiosensitivity principle of malignant tissues (liver metastases and hepatocellular carcinomas are more radiosensitive than normal hepatic parenchyma). Since malignant tissues in the liver preferentially utilize the hepatic artery as their blood supply, intra-arterial internal radiotherapies can achieve significantly higher concentration of radionuclide in the tumors compared to the benign liver parenchyma (which receives 75%-90% of its blood supply from the portal vein). Although these therapies are not molecular targeted, they are lesion selective by virtue of differences in blood supply. Both resin and glass microspheres impregnated with 90Y have been used for selective internal radiation therapy. Even though the radionuclide remains trapped within the microspheres intra-vascularly, the high energy and “relatively” long path length of the emitted beta particles provide good penetration into the immediately adjacent malignant tumor tissue. Resin and glass microspheres are fairly similar in diameter (30-35 um vs. 20-30 um, respectively). However, the activity per particle is significantly higher with glass microspheres (2500 Bq vs. 50 Bq), requiring fewer particles and lower embolic potential to provide similar radiation levels. Another technical difference is that high specific gravity of glass particles requires high pressure during administration compared to resin microspheres.
Extrahepatic adverse effects with this therapy are usually due to arterial collaterals or arteriovenous shunts associated with the tumors or cirrhosis (15). In fact, these tumors tend to recruit vessels from the diaphragm and adjacent organs such as stomach,
duodenum, or pancreas. As a fraction of microspheres enters systemic circulation through these arteriovenous shunts and blood supply of adjacent organs, they may cause pneumonitis, cholecystitis, gastritis, duodenitis, and pancreatitis. A radiation dose of 30 Gy (or a cumulative dose of 50 Gy) to the lung is associated with pneumonitis, and long-term significant morbidity due to eventual development of pulmonary fibrosis. Therefore, prior to intra-arterial radioembolization of liver lesions, the hepatic arterial anatomy has to be mapped with catheter angiography and collaterals to other organs visualized, so that they can be coiled. The degree of hepatopulmonary shunting from the macro and microcirculation also has to be mapped and quantitated prior to hepatic arterial embolization via intra-arterial administration of 99mTc-macro aggregated albumin (MAA) and subsequent imaging. The administered radioactivity by means of microspheres may need to be limited if there is significant shunting to the lungs or if there is any activity in other abdominopelvic viscera that cannot be corrected by coil embolization. In that sense, this practice works in tandem with a diagnostic image that guides the therapeutic component. Although not molecularly targeted or using the same molecular probe for diagnostic and therapeutic purposes, the utility of this combination of diagnostic/therapeutic pair is necessary, used in this case more to avoid undesirable and preventable side effects than to assess the effectiveness of the therapy. In that sense, intra-arterial MAA is a surrogate for microspheres.
duodenum, or pancreas. As a fraction of microspheres enters systemic circulation through these arteriovenous shunts and blood supply of adjacent organs, they may cause pneumonitis, cholecystitis, gastritis, duodenitis, and pancreatitis. A radiation dose of 30 Gy (or a cumulative dose of 50 Gy) to the lung is associated with pneumonitis, and long-term significant morbidity due to eventual development of pulmonary fibrosis. Therefore, prior to intra-arterial radioembolization of liver lesions, the hepatic arterial anatomy has to be mapped with catheter angiography and collaterals to other organs visualized, so that they can be coiled. The degree of hepatopulmonary shunting from the macro and microcirculation also has to be mapped and quantitated prior to hepatic arterial embolization via intra-arterial administration of 99mTc-macro aggregated albumin (MAA) and subsequent imaging. The administered radioactivity by means of microspheres may need to be limited if there is significant shunting to the lungs or if there is any activity in other abdominopelvic viscera that cannot be corrected by coil embolization. In that sense, this practice works in tandem with a diagnostic image that guides the therapeutic component. Although not molecularly targeted or using the same molecular probe for diagnostic and therapeutic purposes, the utility of this combination of diagnostic/therapeutic pair is necessary, used in this case more to avoid undesirable and preventable side effects than to assess the effectiveness of the therapy. In that sense, intra-arterial MAA is a surrogate for microspheres.
Radiation-induced liver disease (RILD) is an uncommon complication that can occur 60 to 90 days posttreatment presenting with jaundice, ascites, and elevated bilirubin levels. Small livers (<1.5 kg) or reduced hepatic reserve in the setting of prior liver-directed therapy, steatosis, steatohepatitis, hepatitis or cirrhosis, and low tumor burden (<5%) are the risk factors associate with RILD. Pretreatment with steroids may reduce the risk.
Radio-theranostics in the clinic
In this section, we focus on the radio-theranostic pairs which are either used currently in the clinic or are anticipated to be approved for use soon. Significant research is currently undergoing to develop new theranostic pairs and expand the clinical radio-theranostic applications. Promising results from multiple clinical trials studying diagnostic/therapeutic radio-probes for various cancers including for example prostate carcinoma (targets such as PSMA and gastrin-releasing peptide receptor), lymphoproliferative malignancies (various targets including chemokine receptor type 4, CD20, and CD37), ovarian and breast cancers (targeting human epidermal growth factor receptor 2) are becoming more and more available.
Table 16.2 COMMONLY USED IODINE ISOTOPES IN THERANOSTICS | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Radioiodine
Radioactive iodine has been used for diagnosis and treatment of benign and malignant thyroid disorders since 1940s, and it is by definition the first radio-theranostic agent used (16). The molecular mechanism is based on the fact that thyroid follicular cells take up iodine (radioactive or non-radioactive) from the blood via the sodium-iodide symporters (NIS) localized in the basolateral membrane, which cotransports two sodium ions along with one iodide ion inside the cell via NIS (17). Thyroid hormone biosynthesis requires iodide efflux through the apical membrane via pendrins into the follicular lumen, with subsequent oxidation, organification, and incorporation in tyrosyl residues of thyroglobulin at the cell-colloid interface. Iodinated thyroglobulin enters the cell via pinocytosis and undergoes hydrolysis, and T3 and T4 are subsequently secreted into the bloodstream at the basolateral membrane (18). NIS is used by the radioiodine family to get inside the cancer cell and follow the subsequent iodine metabolism. This molecular mechanism can be used for diagnostic and therapeutic radioiodine purposes. Of all 37 known iodine isotopes, all except one (127I) are radioactive, and several of which (123I, 124I, 125I, and 131I) are commercially available for diagnostic and therapeutic purposes depending on their emissions (Table 16.2). All of them follow NIS for their diagnostic or therapeutic interactions with benign or malignant thyroid cells.
Thyroid-stimulating hormone (TSH) and iodide modulate NIS activity via transcriptional and posttranscriptional mechanisms. High concentrations of iodide lead to reduction in both NIS mRNA and protein levels and inhibit organification of iodine (Wolff-Chaikoff effect). It is however unclear if a low-iodine diet (defined by an intake of <50 µg/day for 1-2 weeks) can increase NIS expression independent of TSH. The TSH receptor is the major controller of thyroid cell proliferation, differentiation, and function via activation of the cAMP cascade (19). Stimulation of TSH receptors by autoantibodies in Graves’ disease or weak binding by human chorionic gonadotropin (hCG) in gestational hyperthyroidism results in increased iodine uptake.
Several combinations of radioiodine theranostic pairs can be used for the treatment of benign as well as malignant thyroid. 123I is used for diagnosing and radioiodine therapy planning in Graves’ disease, toxic adenoma, and toxic multinodular goiter. These conditions are associated with elevated radioiodine uptake (particularly Graves’ disease) in contrast to other etiologies of hyperthyroidism. Quantification of uptake and retention at 24 h allows for delivery of a precise dose of 131I to the target tissue (e.g., 0.15-0.2 mCi/g [20]).
Radioactive iodine is a critical tool for staging, surveillance, and treatment in well-differentiated thyroid carcinomas.
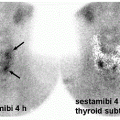
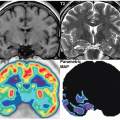
Differentiated thyroid cancer which includes papillary and follicular histology is a common malignancy and has a favorable prognosis and very low mortality compared with most other malignancies. Differentiated thyroid cancer (papillary thyroid carcinoma being the most common comprising 70%-75% of differentiated thyroid carcinomas, followed by follicular thyroid carcinoma in 10%-15% of cases in the United States and 17%-20% in the world) expresses NIS. Hurthle cell carcinoma is an aggressive variant of follicular carcinoma and is associated with low avidity for radioiodine in most patients (21). In general, NIS expression is lower in cancer than in normal thyroid follicular cells and may be even lower in metastatic lesions than in the primary cancer tissue. Therefore, stimulation of TSH release in patients with history of thyroid cancer and total thyroidectomy via withdrawal of thyroid hormone intake, or intramuscular administration of recombinant human TSH (rhTSH, thyrotropin) is used to increase radioiodine uptake in residual/recurrent/metastatic thyroid cancer for diagnostic (123I and 124I) and therapeutic purposes (131I) using theranostic pairs of the iodine family. Poorly differentiated and dedifferentiated thyroid cancer have reduced functional NIS expression at the cellular membrane and loses the ability to trap Iodine. Therefore, the utility of theranostics with radioiodine pairs also decreases. Nevertheless, some medications such as selumetinib are now known to induce re-differentiation of these cancer cells and increase radioiodine uptake again (22). The clinical utility of these medications prior to 124I/131I diagnostic/treatment is being evaluated (23) and already used currently in some Institutions (Fig. 16.1).
Differentiated thyroid cancer which includes papillary and follicular histology is a common malignancy and has a favorable prognosis and very low mortality compared with most other malignancies. Differentiated thyroid cancer (papillary thyroid carcinoma being the most common comprising 70%-75% of differentiated thyroid carcinomas, followed by follicular thyroid carcinoma in 10%-15% of cases in the United States and 17%-20% in the world) expresses NIS. Hurthle cell carcinoma is an aggressive variant of follicular carcinoma and is associated with low avidity for radioiodine in most patients (21). In general, NIS expression is lower in cancer than in normal thyroid follicular cells and may be even lower in metastatic lesions than in the primary cancer tissue. Therefore, stimulation of TSH release in patients with history of thyroid cancer and total thyroidectomy via withdrawal of thyroid hormone intake, or intramuscular administration of recombinant human TSH (rhTSH, thyrotropin) is used to increase radioiodine uptake in residual/recurrent/metastatic thyroid cancer for diagnostic (123I and 124I) and therapeutic purposes (131I) using theranostic pairs of the iodine family. Poorly differentiated and dedifferentiated thyroid cancer have reduced functional NIS expression at the cellular membrane and loses the ability to trap Iodine. Therefore, the utility of theranostics with radioiodine pairs also decreases. Nevertheless, some medications such as selumetinib are now known to induce re-differentiation of these cancer cells and increase radioiodine uptake again (22). The clinical utility of these medications prior to 124I/131I diagnostic/treatment is being evaluated (23) and already used currently in some Institutions (Fig. 16.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree