- •
A key physiologic attribute that defines the quality of a MPI agent is defined by the relationship between the tracer uptake and MBF. A linear relationship maximizes the sensitivity of the tracer to identify flow-limiting coronary artery stenosis.
- •
Cardiac SPECT perfusion tracers include 201 Tl and the 99m Tc-labeled tracers, tetrofosmin and sestamibi.
- •
201 Tl has better extraction than 99m Tc-labeled tracers at high blood flow rates, but its lower photon energy and higher radiation dose make it less ideal for clinical SPECT imaging.
- •
99m Tc-labeled tracers’ photon energies (140 keV) are ideal for clinical SPECT systems. Their more favorable organ dosimetry allows for higher injected doses, resulting in less attenuation artifacts and improved image quality.
- •
Clinical radiotracers for PET MPI include 82 Rb and 13 N-ammonia.
- •
82 Rb has a very short half-life and can only be used with pharmacologic stress testing.
- •
13 N-ammonia can be used with pharmacologic- or exercise-stress testing.
- •
New PET tracers for myocardial perfusion and delivery systems may offer further advantages for PET MPI in the near future.
- •
MPI protocols should be tailored based on the patient’s history and imaging equipment to maximize image quality while minimizing radiation exposure.
- •
Stress-only imaging is one of the most effective protocols for reducing radiation exposure and is best suited for patients without a history of CAD.
- •
99m Tc-PYP is an effective radiotracer for the detection of ATTR amyloid cardiomyopathy.
- •
123 I-mIBG is a norepinephrine analog that is approved for the imaging of cardiac sympathetic nerve function in patients with heart failure.
Introduction
Radioactive tracers for cardiac imaging have evolved significantly since the 1970s when Zaret and Strauss used potassium (K)-43 to noninvasively assess myocardial perfusion after treadmill exercise. Although development of new radioactive tracers for cardiac imaging was relatively static from the mid-1990s on, there have been a number of recent advancements in the development and delivery of radioactive tracers that bring new options to modern nuclear cardiology.
The most common application of radioactive tracers in cardiac imaging is for the assessment of myocardial perfusion. A key concept to optimally use and interpret radionuclide perfusion images is the understanding of the ideal physiologic characteristics of radioactive tracers for the assessment of myocardial tissue perfusion. These characteristics have been well described and combine five key properties:
- 1.
High myocardial uptake;
- 2.
High first-pass extraction with a linear relationship between myocardial radiotracer uptake and myocardial blood flow (MBF);
- 3.
High target-to-background ratio to avoid imaging contamination from adjacent organs;
- 4.
Ability to quantify MBF; and
- 5.
Transport of the radiotracer must track the physiologic effects of altered blood flow.
As shown in Fig. 4.1 , current myocardial perfusion radiotracers have varying degrees of nonlinear relationships between their tissue uptake relative to actual MBF. The lack of linearity between myocardial uptake and blood flow (so-called “roll off”), especially at high flow rates, has a direct impact on the degree of heterogeneity in radiotracer tissue retention, which, in turn, affects the sensitivity of the test for uncovering varying degrees of coronary stenoses. For example, 99m technetium-labeled myocardial perfusion radiotracers show a marked roll off of uptake at higher blood flow rates that explains, in part, their relatively lower sensitivity for evaluating the functional significance of coronary stenosis of intermediate severity (50% to 70%).

Despite nuclear cardiology’s history and foundation in myocardial perfusion imaging, the field is rapidly evolving to include other relevant imaging targets in clinical medicine, including cardiac sympathetic innervation and amyloidosis, which are changing disease management. Novel radioactive tracers for myocardial perfusion imaging with positron emission tomography (PET) are currently in clinical trials. In addition, there are advances in production and delivery systems of PET perfusion radiotracers that are improving clinical access to cardiac PET imaging.
This chapter will review commonly used radioactive tracers for single photon emission computed tomography (SPECT) and PET imaging of the heart, primarily for the assessment of perfusion but also for imaging of nonperfusion targets.
Current radiotracers for cardiac imaging
SPECT radiotracers
Radionuclide myocardial perfusion imaging was developed in the 1970s and included the rapid clinical development of 201 thallium ( 201 Tl) with its U.S. Food and Drug Administration (FDA) approval in 1977 as a myocardial perfusion tracer. Although it possesses good physiologic properties for tracking MBF, 201 Tl has radiophysical properties that limit its use, including high radiation exposure that limits injected doses and low photon energy, both of which make imaging challenging, especially in obese patients. These limitations led to the development and commercialization of 99m technetium ( 99m Tc)-labeled lipophilic cations, specifically sestamibi (FDA-approved in 1990) and tetrofosmin (FDA-approved in 1996). Despite the suboptimal relationship between their myocardial tracer uptake and blood flow, the 99m Tc-labeled tracers have earned a dominant place in modern nuclear cardiology practice. In addition to the myocardial perfusion imaging (MPI) tracers, 99m Tc-pyrophosphate (PYP) and 123 I-meta-iodobenzylguanidine ( 123 I-mIBG) are commonly used for cardiovascular molecular imaging of transthyretin (ATTR) cardiac amyloidosis and sympathetic innervation, respectively. An overview of radiotracers for cardiac SPECT is summarized in Table 4.1 .
| Tracer | Physical Half-life (in hours) | Primary Photon Energy | Source | Uptake | Myocardial Clearance | Redistribution | Maximum Extraction Fraction |
| 201 Thallium | 73 | 68–82 keV | Cyclotron | Active; Na/K ATPase | 50% at 6 hours | Yes | 85% |
| 99m Technetium-sestamibi | 6.02 | 140 keV | Generator | Passive; mitochondrial membrane potential | Minimal | Minimal | 55%–65% |
| 99m Technetium-tetrofosmin | 6.02 | 140 keV | Generator | Passive; mitochondrial membrane potential | Minimal | Minimal | 50%–55% |
| 99m Technectiumpyrophosphate | 6.02 | 140 keV | Generator | Extracellular calcium | Minimal | None | NA |
| 123 Iodine | 13.2 | 159 keV | Cyclotron | Active | Minimal | None | NA |
201 Thallium
201 Tl is produced in a cyclotron from the proton-bombardment of nonradioactive 203 Tl, lead, and/or bismuth and decays via a 68- to 82-keV mercury characteristic x-ray emission. 201 Tl can also be produced from 201 lead (half-life of 9.3 hours) by a generator-based method. Because of its long half-life (73 hours), 201 Tl is usually transported from the cyclotron to the end user.
Mechanism of retention and property of redistribution
201 Tl is a potassium analog whose initial cellular uptake is facilitated by the sarcolemmal adenosine triphosphate (ATP)-requiring Na+/K+ ATPase. Like most perfusion tracers, initial 201 Tl uptake is proportional to MBF at low/intermediate flow rates with “roll off” and loss of proportionality to flow at higher flow rates (see Fig. 4.1 ). Myocardial first-pass extraction of 201 Tl is superior to that of the 99m Tc-labeled agents, meaning smaller differences in MBF are required to visualize differential tracer uptake relative to flow and potentially increasing sensitivity for less severe ischemia. In addition, 201 Tl exhibits biexponential clearance, also known as redistribution , which allows for delayed imaging to evaluate for chronically hypoperfused, but still viable, myocardium. In simplistic terms, normally perfused regions of the myocardium have a faster rate of washout than ischemic regions, allowing for the use of late (4 or 24 hours postinjection) redistribution images to define areas of myocardial hibernation and/or viability. Limitations of 201 Tl include its long half-life, which leads to high effective radiation exposure and limits the dose that may be given compared with other perfusion radiotracers. In addition, the relatively low photon energy of 201 Tl makes it less ideal for imaging patients where nonuniform attenuation is present (e.g., obesity, large breast, diaphragmatic attenuation).
99m Technetium-based radiotracers
99m Tc can be produced on site using a commercially available 99 molybdenum ( 99 Mo) generator, a fission reaction product of 235 uranium ( 235 U) produced in a nuclear reactor. 99 Mo lybdenum slowly undergoes β- decay (half-life of 66 hours) with 87.5% of the decay product being 99m Tc. The “m” superscript refers to a metastable excited state that is an intermediate between the excited and stable nuclear states. 99m Technetium produces a 140-keV gamma emission by isomeric transition to produce the stable daughter, 99 technetium. The generator prepared at the radiopharmaceutical manufacturer includes 99 Mo tightly bound to an alumina (Al 2 O 3 ) column. 99m Tc can be eluted (“milked”) from the column with normal saline as 99m Tc-pertechnetate into a collection vial. 99m Tc can then be chemically linked to sestamibi or tetrofosmin using commercial kits or acquired through commercial radiopharmacies in prebound unit doses.
In recent years, significant issues have arisen with worldwide molybdenum-99m supply. Starting in 2007, aging research reactors across the world that are the major producers of 99 Mo have experienced frequent shutdowns, causing critical supply chain disturbances in 2009 and 2010. This led to worldwide shortages of 99 Mo and, consequently, 99m Tc, prompting the need to seek alternative imaging agents for radionuclide perfusion imaging (e.g., 82 rubidium [ 82 Rb] and 13 N-ammonia). Current international multigovernmental and industry approaches are working to create a sustainable 99 Mo supply chain for the coming decades.
99m Technetium has a 6.02-hour half-life, permitting higher doses to be used with lower radiation exposure compared with 201 Tl, which reduces photon scatter and improves image quality, particularly when soft tissue attenuation is present. Because of the superior image quality afforded by the higher energy emission (140 keV for 99m Tc vs. 60 to 80 keV for 201 Tl) and the lower effective radiation dose, the 99m Tc agents have largely supplanted 201 Tl for routine evaluation of myocardial perfusion with SPECT.
Mechanism of retention
99m Technetium-labeled sestamibi and tetrofosmin are lipophilic cations that exhibit MBF–dependent first-pass extraction and are retained in proportion to mitochondrial membrane potential. Both are rapidly cleared from blood early (within 10 minutes) after intravenous (IV) injection and are predominately cleared by hepatobiliary metabolism, which can cause significant uptake adjacent to the heart in many patients and cause significant artifacts, which reduce sensitivity and specificity, as discussed in greater detail in Chapter 5 . 99m Technetium-tetrofosmin has a shorter biologic half-life but greater heart-to-liver uptake ratio compared with sestamibi. ,
99m Technetium-Pyrophosphate
99m Technetium-PYP was originally developed and approved for bone scintigraphy and historically used to detect acute myocardial infarction but has recently been repurposed as an imaging agent for cardiac ATTR amyloidosis (see discussion in Chapter 24 ). PYP is supplied as a kit that includes sodium PYP and stannous chloride reconstituted with raw 99m Tc sodium pertechnetate. The mechanism of retention of PYP appears to rely on local increases in extracellular calcium common in both acute myocardial infarction and ATTR amyloid deposits.
123 I-Meta-Iodobenzylguanidine
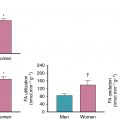
Increased myocardial sympathetic activity is present in heart failure and is an independent predictor of prognosis and poor outcomes, including sudden cardiac death independent of left ventricular (LV) function. Myocardial sympathetic innervation can be assessed using 123 I-meta-iodobenzylguanidine ( 123 I-mIBG) cardiac imaging. The ratio of heart to mediastinal 123 I-mIBG uptake (H:M ratio) has been demonstrated to improve risk prediction in validated clinical variable-based models as a low myocardial 123 I-mIBG uptake (and low H:M ratio) identifies high-risk patients and a H:M ratio greater than 1.6 imparts a low risk. 123 I-mIBG is FDA-approved for the assessment of myocardial sympathetic innervation and mortality risk with patients with New York Heart Association Class II/III heart failure and an LV ejection fraction equal to or less than 35%. Please see detailed discussion on the application of cardiac sympathetic nerve imaging in Chapter 21 .
Production
123 Iodine is produced in a cyclotron and decays via electron capture to 123 Te, producing a 159-keV photon. 123 Imeta-iodobenzylguanidine is compounded as iobenguane sulfate at a radiopharmacy.
Mechanism of retention
123 I-meta-iodobenzylguanidine is structurally similar to norepinephrine and its uptake in presynaptic adrenergic nerve endings involves norepinephrine transport systems discussed in detail in Chapter 21 . Thyroid blockade with potassium iodide of Lugol solution is necessary in most patients before administration of 123 I-mIBG to prevent thyroid uptake of 123 I.
PET radiotracers
There are currently two FDA-approved PET myocardial perfusion radiotracers, 82 Rb and 13 N-ammonia ( Table 4.2 ). Because of its on-site generator-based production, 82 Rb has been the dominant radiotracer for PET MPI over the past 20 years. Nevertheless, new delivery methods for 82 Rb and 13 N-ammonia offer unique possibilities for cardiac PET perfusion imaging, including more reproducible MBF quantification with constant activity 82 Rb delivery systems as well as the ability to perform exercise PET imaging with 13 N-ammonia. In addition, a novel 18 F-labeled perfusion tracer, 18 F-flurpiridaz, is under active Phase III clinical trial investigation for possible forthcoming regulatory approval.
| Tracer | Physical Half-life | Positron Range | Source | Uptake/Retention | First-Pass Extraction Fraction |
| 82 Rubidium | 73 sec | 8.6 | Generator | Active; Na/K ATPase | 65% |
| 13 N-Ammonia | 9.9 min | 2.5 | Cyclotron | Enzymatic conversion to glutamate | 82% |
| 18 F-Fluorodeoxyglucose | 110 min | 1.03 | Cyclotron | GLUT transporters | NA |
| 18 F-Flurpiridaz | 110 min | 1.03 | Cyclotron | Passive; mitochondrial complex 1 | 94% |
82 Rubidium
It is the most commonly used PET tracer for MPI and is produced from the electron capture decay of cyclotron-produced 82 strontrium (half-life of 25.3 days). Similar to a 99m Tc generator, 82 strontium is adsorbed on a shielded column (stannic oxide) and 82 Rb is eluted from the generator with normal saline. The generator eluate ( 82 Rb) is infused directly into the patient’s IV line because the half-life of 82 Rb is short (75 seconds), which has the advantage of reduced radiation exposure and is useful for rapid sequential rest and pharmacologic stress imaging. The short half-life of 82 Rb does not allow for its use with exercise stress protocols.
There are currently two generator infusion systems approved for clinical use in the United States ( Fig. 4.2 ). The RUBY Rubidium Elution System (RbES) is designed for use with the Jubilant DraxImage RUBY-FILL® Rubidium Rb-82 Generator, whereas the Bracco CardioGen-82® (Rubidium Rb-82 Generator) is a closed system designed to be used with the Bracco infusion system. Both are used to produce 82 Rb for IV administration. The CardioGen system offers bolus infusions, whereas the RUBY-FILL system offers two modes of patient infusion: Bolus (either using Constant Flow or Constant Time) and Constant Activity. With the Constant Activity infusion method, the system delivers the dose in a square-wave infusion profile, as opposed to the traditional bolus method. With the constant activity method, the system delivers reproducible infusion delivery profiles regardless of the age of the generator and improves test-retest repeatability of MBF quantification with 82 Rb dynamic PET, as discussed in Chapter 3 .

Mechanism of retention
82 Rubidium is a potassium analog with an extraction fraction similar to thallium but superior to both of the 99m Tc-labeled SPECT radiotracers.
13 N-ammonia
13 N-ammonia was first produced by Joliot and Curie in 1934 and its use in PET MPI may be more advantageous than 82 Rb because of its higher myocardial extraction (up to 80%), which leads to increased sensitivity for ischemia. Its main limitation, however, has been the requirement of an on-site cyclotron, which has precluded its widespread use. Recently, novel “bench top” cyclotrons have become commercially available, potentially allowing more widespread clinical use of 13 N-ammonia. This commercially available minicyclotron with automated 13 N-ammonia production allows on-site production of 13 N-ammonia without the need for a larger cyclotron and radiopharmacy, which are generally required for 13 N-ammonia production.
Ionetix, Inc. has developed a first-of-its-kind cyclotron with improvements over conventional cyclotron technology. The ION-12 SC ( Fig. 4.3 ) is a 12 MeV, 10 μA superconducting cyclotron that uses a novel design with superconducting magnets to shrink its footprint. It can provide consistent and reliable 13 N-ammonia production at the point of care.

Mechanism of retention
After IV injection, it undergoes rapid blood pool clearance with diffusion across cell membranes and trapping inside the cardiomyocyte after irreversible enzymatic conversion to glutamic acid.
18 F-fluorodeoxyglucose
Glucose metabolism in the heart is a highly regulated process of uptake, phosphorylation, and metabolism. Glucose uptake in the heart involves facilitated diffusion down its concentration gradient and entry across the sarcolemma via glucose transporters (GLUTs). Alterations in glucose cardiomyocyte metabolism occur during myocardial ischemia, as well as in inflammatory cells during infection and inflammation. These changes in glucose uptake can be imaged using 18 F-fluorodeoxyglucose ( 18 F-FDG) PET for myocardial viability, cardiac device infections, and myocardial inflammation/sarcoidosis, as discussed in detailed in Chapters 20 and 29 .
Mechanism of retention and production
2-Deoxy-D-glucose (DG) is an isomer of glucose that, after uptake via GLUTs, cannot progress past the initial step of glycolysis. Therefore, after phosphorylation by hexokinase, DG is trapped in the cell. The fluorination of DG with cyclotron-produced 18 F via automated synthesis leads to the production of 18 F-FDG.
18 F-flurpiridaz
18 F-flurpiridaz is an investigational PET perfusion tracer that has completed one series of phase III trials with another phase III trial underway. It exhibits superior first-pass cardiac extraction to 82 Rb, 13 N-ammonia, and SPECT radiotracers, being exceeded only by 15 O-water. The use of an 18 F label, which has a 108-minute half-life, means flurpiridaz can be synthesized regionally, provided in unit doses (like 18 F-FDG), and used in exercise stress testing protocols. As discussed in Chapter 2 , 18 F has a shorter positron range than 82 Rb ( Fig. 4.4 ), improving spatial resolution, which could be of particular benefit in imaging thinned-wall dilated ventricles and increasing the sensitivity to small defects. Having near-ideal first-pass extraction without the unique practical challenges of 15 O-water, 18 F-flurpiridaz promises a balance between excellent sensitivity, accurate MBF quantification, and clinical feasibility. MBF reserve measurement with 18 F-flurpiridaz has been validated in humans and clinical trials. ,

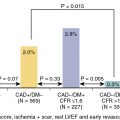
Data from the first phase III trial showed that 18 F-flurpiridaz was more sensitive for coronary artery disease (CAD) detection compared with 99m Tc-SPECT MPI (71.9% vs. 53.7%; p < 0.001). Nevertheless, it did not achieve its prespecified second primary outcome of noninferiority in specificity compared with SPECT MPI (72.6% vs. 82.6%; p = 0.9450), despite higher diagnostic certainty ( p < 0.001) and superior image quality ( p < 0.001). Currently, a second phase III trial is underway (ClinicalTrials.gov identifier: NCT03354273).
Imaging protocols for cardiac SPECT and PET
Nuclear cardiology imaging acquisition protocols have evolved tremendously in recent years from a traditional rest/stress imaging protocol to now focus on best practices that optimize accuracy, safety, and radiation exposure. These best practices for cardiac imaging combine protocols to reduce radiation exposure by using stress-only imaging, techniques such as multiposition (prone) imaging or, in some cases, hybrid nuclear-CT imaging to compensate for nonuniform attenuation, and the use of advanced detector technologies (such as solid-state detector SPECT; see also Chapters 5 and 6 ). The optimal imaging protocols for each laboratory and patient should be individualized, based on imaging equipment, stress modalities, reader experience, patient characteristics, and the clinical question needing to be answered.
This focus on nuclear cardiology best practices has been codified in a series of position statements that include goals for reducing the average radiation exposure to less than 9 mSv per study, using specific imaging and stress strategies to optimally choose the best test for each patient (so-called patient-centered imaging), and defining standards of care to optimize results.
SPECT imaging protocols
Table 4.3 includes the recommendations from the American Society of Nuclear Cardiology 2018 SPECT Imaging Guidelines for 99m Tc SPECT imaging using traditional Anger cameras for either a 1- or 2-day imaging protocol. It is especially important to be mindful of the recommended 3:1 ratio of radiotracer dose for the second study compared with the first study to optimize sensitivity and image quality. In addition, the preferential recommendation of noncircular orbits and 128 × 128 matrices are important parameters to optimize image quality and resolution. These acquisition protocols are summarized in Fig. 4.5 . In addition, the use of body mass index (BMI)–based or weight-based dosing strategies and stress first/stress only imaging protocols are increasingly recognized as important methods to reduce radiation exposure from MPI studies (see also Chapters 5 and 6 ). Lastly, the use of solid-state detector CZT SPECT cameras can enable an around 50% dose reduction because of their increased sensitivity. The recommendations for CZT SPECT acquisition protocols for 99m Tc SPECT imaging are listed in Table 4.4 .