Radioprotectors
▪ THE DISCOVERY OF RADIOPROTECTORS
Some substances, although they do not directly affect the radiosensitivity of cells, nevertheless, may protect whole animals because they cause vasoconstriction or, in some way, upset normal processes of metabolism to such an extent that the oxygen concentration in critical organs is reduced. Because cells are less sensitive to x-rays under hypoxia, this confers a measure of protection. Examples of such protective substances are sodium cyanide, carbon monoxide, epinephrine, histamine, and serotonin. Such compounds are not really radioprotectors per se and are not discussed further here.
The most remarkable group of true radioprotectors is the sulfhydryl (SH) compounds. The simplest is cysteine, an SH compound containing a natural amino acid, the structure of which is
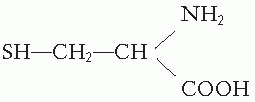
In 1948, Patt discovered that cysteine could protect mice from the effects of total body exposure to x-rays if the drug was injected or ingested in large amounts before the radiation exposure. At about the same time, Bacq and his colleagues in Europe independently discovered that cysteamine could also protect animals from total body irradiation. This compound has a structure represented by
Animals injected with cysteamine to concentrations of about 150 mg/kg require doses of x-rays 1.8 times larger than control animals to produce the same mortality rate. This factor of 1.8 is called the dose reduction factor (DRF), defined as
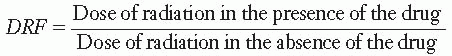
to produce a given level of lethality.
▪ MECHANISM OF ACTION
Many similar SH compounds have been tested and found to be effective as radioprotectors. The most efficient SH compounds tend to have certain structural features in common: a free SH group (or potential SH group) at one end of the molecule and a strong basic function, such as amine or guanidine, at the other end, separated by a straight chain of two or three carbon atoms. SH compounds are efficient radioprotectors against sparsely ionizing radiations such as x- or γ-rays.
The mechanisms most implicated in SH-mediated cytoprotection include:
Free-radical scavenging that protects against oxygen-based free radical generation by ionizing radiations or chemotherapy agents such as alkylating agents
Hydrogen atom donation to facilitate direct chemical repair at sites of DNA damage
Chapter 1 includes a discussion of the chain of events between the absorption of a photon and the eventual biologic damage, which includes the
production of free radicals, which are highly reactive species. If these free radicals are scavenged before they can interact with biologic molecules, the effect of the radiation is reduced. This process is illustrated in Figure 9.1.
production of free radicals, which are highly reactive species. If these free radicals are scavenged before they can interact with biologic molecules, the effect of the radiation is reduced. This process is illustrated in Figure 9.1.
The protective effect of SH compounds tends to parallel the oxygen effect, being maximal for sparsely ionizing radiations (e.g., x- or γ-rays) and minimal for densely ionizing radiations (e.g., low-energy α-particles). It might be predicted that with effective scavenging of all free radicals, the largest possible value of DRF for sparsely ionizing radiations would equal the oxygen enhancement ratio, with a value of 2.5 to 3.0.
TABLE 9.1 Effect of Adding a Phosphate-Covering Function on the Free Sulfhydryl of β-Mercaptoethylamine (MEA) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
This simple description of the mechanism of action of SH radioprotectors is intellectually satisfying, but it is clearly not the whole story because radioprotectors of this class have more effect with densely ionizing radiations (such as neutrons) than would be expected based on this explanation alone. Other factors must be involved that are not fully understood.
▪ DEVELOPMENT OF MORE EFFECTIVE COMPOUNDS
The discovery in 1948 of a compound that offered protection against radiation excited the interest of the U.S. Army because the memory of Nagasaki and Hiroshima was vivid in the years immediately after World War II. However, although cysteine is a radioprotector, it is also toxic and induces nausea and vomiting at the dose levels required for radioprotection. A development program was initiated in 1959 by the U.S. Army in studies conducted at the Walter Reed Institute of Research to identify and synthesize drugs capable of conferring protection to individuals in a radiation environment, but without the debilitating toxicity of cysteine or cysteamine. More than 4,000 compounds were synthesized and tested. At an early stage, the important discovery was made that the toxicity of the compound could be greatly reduced if the SH group was covered by a phosphate group. This is illustrated for cysteamine, otherwise known as mercaptoethylamine (MEA), in Table 9.1. The 50% lethal
dose of the compound in animals can be doubled and the protective effect in terms of the DRF can be greatly enhanced if the SH group is covered by a phosphate. This tends to reduce systemic toxicity. Once in the cell, the phosphate group is stripped and the SH group begins scavenging for free radicals.
dose of the compound in animals can be doubled and the protective effect in terms of the DRF can be greatly enhanced if the SH group is covered by a phosphate. This tends to reduce systemic toxicity. Once in the cell, the phosphate group is stripped and the SH group begins scavenging for free radicals.
TABLE 9.2 Two Radioprotectors in Practical Use |
|---|