Rectal cancer stages
TNM staging
Dukes staging
5-year survival
Stage I
T1-2 N0 M0
A
>90 %
Stage II
A
T3 N0 M0
B
60–85 %
B
T4 N0 M0
60–85 %
Stage III
A
T1-2 N1 M0
C
55–60 %
B
T3-4 N1 M0
35–42 %
C
T1-4 N2 M0
25–27 %
Stage IV
T1-4 N0-2 M1
5–7 %
The TNM stage – dependent 5-year survival rate for rectal carcinomas is as follows [17]:
Stage I – 90 %
Stage II – 60–85 %
Stage III – 27–60 %
Stage IV – 5–7 %
13.8 Medical Care
The surgical definition of the rectum differs from the anatomical definition; surgeons define the rectum as starting at the level of the sacral promontory, while anatomists define the rectum as starting at the level of the third sacral vertebra. Therefore, the measured length of the rectum varies from 12 to 15 cm. The rectum is different than the rest of the colon, in that the outer layer is made of longitudinal muscle. The rectum contains threefolds, namely valves of Houston. The superior (10–12 cm) and inferior (4–7 cm) folds are located on the left side and middle fold (8–10 cm) is located at the right side.
Determination of optimal treatment plan for patients with rectal cancer involves a complex decision-making process. Strong considerations should be given to the intent of surgery, possible functional outcome, and preservation of anal continence and genitourinary functions. The first step involves achievement of cure because the risk of pelvic recurrence is high in patients with rectal cancer and locally recurrent rectal cancer has a poor prognosis. Functional outcome of different treatment modalities involves restoration of bowel function with acceptable anal continence and preservation of genitourinary functions. Preservation of both anal and rectal reservoir function in treatment of rectal cancer is highly preferred by patients. Sphincter-saving procedures for rectal cancer are now considered the standard of care [18].
Factors influencing sphincter and organ preservation in patients with rectal cancer can be described as follows [18]:
Factors influencing sphincter preservation: surgeon training, surgeon volume, neoadjuvant chemoradiotherapy.
Factors associated with difficult sphincter preservation: male sex, morbid obesity, preoperative incontinence, direct involvement of anal sphincter muscles with carcinoma, bulky tumors within 5 cm from the anal verge.
Patient selection for local excision: lesions located in low rectum (within 8–10 cm), lesions occupying less than one third of the rectal circumference, mobile exophitic or polypoid lesions, lesions less than 3 cm in size, T1 lesions, low grade tumor (well or moderately differentiated), negative nodal status (clinical and radiographic).
Disadvantages of APR: need for permanent colostomy, significantly higher short-term morbidity and mortality, significantly higher long-term morbidities, higher rate of sexual and urinary dysfunction.
13.9 Surgical Care
Patient-related, tumor-related, treatment-, and surgeon-related factors influence the ability to restore intestinal continuity in patients with rectal cancer.
13.9.1 Transanal Excision
The local transanal excision of rectal cancer is reserved for early-stage cancers in a select group of patients. The lesions amenable for local excision are small (<3 cm in size), occupying less than a third of a circumference of the rectum, preferably exophytic/polypoid, superficial and mobile (T1 and T2 lesions), low-grade tumors (well or moderately differentiated) that are located in low in the rectum (within 8 cm of the anal verge). There should also be no palpable or radiologic evidence of enlarged mesenteric lymph nodes. The likelihood of lymph node involvement in this type of lesion ranges from 0 % to 12 % [18, 19]. A study by Peng et al. found that local excision in early stage rectal cancer may result in high local recurrence rates. The authors recommend only using this procedure in highly selective groups of patients, specifically those with a tumor size of 2.5 cm or smaller [20].
Local excision is increasingly used to treat stage I rectal cancers despite its inferiority to total mesorectal excision, which is the current standard of care. In a study of all rectal cancer patients in the National Cancer Data Base from 1998 to 2010, researchers found that local excision was used to treat 46.5 % of the patients with T1 tumors and 16.8 % of those with T2 tumors. For patients with T1 cancer, local excision rates increased from 39.8 % in 1998 to 62.0 % in 2010. For patients with T2 cancers, rates increased from 12.2 % to 21.4 % [21].
Preoperative ERUS should be performed. If nodes are identified as suggestive of cancer, do not perform transanal excision. The lesion is excised with the full thickness of the rectal wall, leaving a 1-cm margin of normal tissue. The defect is usually closed; however, some surgeons leave it open. Unfavorable pathologic features such as positive resection margins, lymphovascular invasion, lymph node metastasis, perineural invasions, and recurrent lesion at follow-up evaluations mandate salvage resection. Usually, an abdominal perineal resection or proctosigmoidectomy with coloanal anastomosis is performed as a salvage resection following failure of local excision [19].
The advantages of local excision include rapid recovery, minimal effect on sphincter function, and relatively low perioperative morbidity and mortality. Recovery is usually rapid. The 5-year survival rate after transanal excision ranges from 65 % to 100 % (these figures include some patients with T2 lesions). The local recurrence rate ranges from 0 % to 40 %. Patients with lesions that display unfavorable histologic features but are excised completely may be treated with adjuvant radiation therapy.
Cancer recurrence following transanal excision of early rectal cancer has been studied by Weiser et al. [22]. Failures due to transanal excision are mostly advanced local disease and are not uniformly salvageable with radical pelvic excision. These patients may require extended pelvic dissection with en bloc resection of adjacent pelvic organs such as the pelvic side wall with autonomic nerves, coccyx, prostate, seminal vesicle, bladder, vagina, ureter, ovary, and uterus. The long-term outcome in patients with recurrent rectal carcinoma who undergo radical resection is less favorable than expected, relative to the early stage of their initial rectal carcinoma [22].
In summary, the treatment of T1 and T2 rectal cancers continues to be challenging. Local excision is associated with higher rate of recurrence, especially in T2 lesions. Ultimately, 15–20 % of patients may experience recurrence. When local recurrence is detected, patients usually have advanced disease, requiring extensive pelvic excisions. Therefore, strict selection criteria are essential when considering local excision. All patients should be informed of the risk of local recurrence and lower cure rates associated with recurrence [18, 22, 23].
13.10 Endocavitary Radiation
This radiotherapy method differs from external-beam radiation therapy in that a larger dose of radiation can be delivered to a smaller area over a shorter period. Selection criteria for this procedure are similar to those for transanal excision. The lesion can be as far as 10 cm from the anal verge and no larger than 3 cm. Endocavitary radiation is delivered via a special proctoscope and is performed in an operating room with sedation. The patient can be discharged on the same day.
A total of six application of high-dose (20–30 Gy), low-voltage radiation (50 kV) is given over the course of 6 weeks. Each radiotherapy session produces a rapid shrinkage of the rectal cancer lesion. An additional booster dose can be given to the tumor bed. The overall survival rate is 83 %, although the local recurrence rate as high as 30 % [19].
13.11 Transanal Endoscopic Microsurgery (TEM)
Transanal endoscopic microsurgery is another form of local excision that uses a special operating proctoscope that distends the rectum with insufflated carbon dioxide and allows the passage of dissecting instruments. This method can be used on lesions located higher in the rectum and even in the distal sigmoid colon. Transanal endoscopic microsurgery has not come into wide use yet because of a significant learning curve and a lack of availability.
13.12 Sphincter-Sparing Procedures
Procedures are described that use the traditional open technique. All of these procedures, except the perineal portions, can also be performed using laparoscopic techniques, with excellent results. The nuances of the laparoscopic technique used are beyond the scope of this discussion. A study by Li et al. found that laparoscopic and open surgery for middle and lower rectal cancer are associated with similar long-term outcomes. The study shows the value of technical experience when performing laparoscopic surgery and encourages the use of this surgery by experienced teams [24]. Long-term results from the UK Medical Research Council trial of laparoscopically assisted versus open surgery for colorectal cancer showed no differences between groups in overall or disease-free survival or recurrence rates [25].
13.12.1 Low Anterior Resection (LAR)
LAR is generally performed for lesions in the middle and upper third of the rectum and, occasionally, for lesions in the lower third. Because this is a major operation, patients who undergo LAR should be in good health. They should not have any preexisting sphincter problems or evidence of extensive local disease in the pelvis.
Patients will not have a permanent colostomy but should be informed that a temporary colostomy or ileostomy may be necessary. They also must be willing to accept the possibility of slightly less-than-perfect continence after surgery, although this is not usually a major problem.
Other possible disturbances in function include transient urinary dysfunction secondary to weakening of the detrusor muscle. This occurs in 3–15 % of patients. Sexual dysfunction is more prominent and includes retrograde ejaculation and impotence. In the past, this has occurred in 5–70 % of men, but recent reports indicate that the current incidence is lower [26].
The operation entails full mobilization of the rectum, sigmoid colon, and, usually, the splenic flexure. Mobilization of the rectum requires a technique called total mesorectal excision (TME). TME involves sharp dissection in the avascular plane that is created by the envelope that separates the entire mesorectum from the surrounding structures (Fig. 13.1). This includes the anterior peritoneal reflection and Denonvilliers fascia anteriorly and preserves the inferior hypogastric plexus posteriorly and laterally. TME is performed under direct visualization. Mesorectal spread can occur by direct tumor spread, tumor extension into lymph nodes, or perineural invasion of tumor [14, 23, 26].
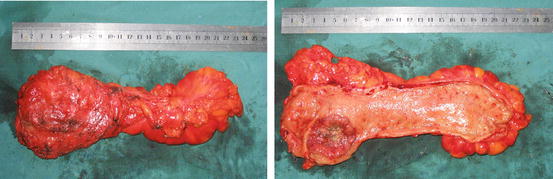

Fig. 13.1
The specimen of rectum after TME resection (Courtesy of Jun Li, MD, Second Affiliated Hospital Zhejiang University School of Medicine)
TME yields a lower local recurrence rate (4 %) than transanal excision (20 %), but it is associated with a higher rate of anastomotic leak (11 %). For this reason, TME may not be necessary for lesions in the upper third of the rectum. The distal resection margin varies depending on the site of the lesion. A 2-cm margin distal to the lesion must be achieved. For the tumors of the distal rectum, less than 5 cm from the anal verge, the minimally accepted distal margin is 1 cm in the fresh specimen. Distal intra-mural spread beyond 1 cm occurs rarely. Distal spread beyond 1 cm is associated with aggressive tumor behavior or advanced tumor stage [14].
The procedure is performed with the patient in the modified lithotomy position with the buttocks slightly over the edge of the operating table to allow easy access to the rectum [23] (See the table below). A circular stapling device is used to create the anastomosis. A double-stapled technique is performed. This entails transection of the rectum distal to the tumor from within the abdomen using a linear stapling device. The proximal resection margin is divided with a purse-string device.
After sizing the lumen, the detached anvil of the circular stapler is inserted into the proximal margin and secured with the purse-string suture. The circular stapler is inserted carefully into the rectum, and the central shaft is projected through or near the linear staple line. Then, the anvil is engaged with the central shaft, and, after completely closing the circular stapler, the device is fired. Two rings of staples create the anastomosis, and a circular rim or donut of tissue from the proximal and distal margins is removed with the stapling device.
According to a study by Maurer et al. the introduction of TME has resulted in an impressive reduction of local recurrence rate. TME appears to have improved survival in patients without systemic disease [27] (Table 13.2).
Resection margins | Proximal resection margin (cm) | Distal resection margin (cm) |
|---|---|---|
Ideal margins | 5 cm or more | 2 cm or more |
Minimally acceptable margins | 5 cm or more | 1 cm or more |
The anastomotic leak rate with this technique ranges from 3 % to 11 % for middle-third and upper-third anastomosis and to 20 % for lower-third anastomosis. For this reason, some surgeons choose to protect the lower-third anastomosis by creating a temporary diverting stoma. This is especially important when patients have received preoperative radiation therapy. The rate of stenosis is approximately 5–20 %. A hand-sewn anastomosis may be performed; if preferred, the anastomosis is performed as a single-layer technique. The leak and stenosis rates are the same.
In R0 resection, the inferior mesenteric artery (IMA) should be excised at its origin, but this rule is not mandated by available supportive evidence. Patients with non–en-bloc resection, positive radial margins, positive proximal and distal margin, residual lymph node disease, and incomplete preoperative and intra-operative staging would not be considered to have complete resection of cancer (R0 resection) [14]. Patients with R1 and R2 resection are considered to have an incomplete resection for cure. Incomplete R1 and R2 resection does not change the TNM stage but affects the curability [14]. In a 2012 multicenter, randomized controlled trial, mesorectal excision with lateral lymph node dissection was associated with a significantly longer operation time and significantly greater blood loss than mesorectal excision alone [28].
13.12.2 Colo-anal Anastomosis (CAA)
Very distal rectal cancers that are located just above the sphincter occasionally can be resected without the need for a permanent colostomy. The procedure is as already described; however, the pelvic dissection is carried down to below the level of the levator ani muscles from within the abdomen. A straight-tube coloanal anastomosis (CAA) can be performed using the double-stapled technique, or a hand-sewn anastomosis can be performed transanally [26].
The functional results of this procedure have been poor in some patients, who experience increased frequency and urgency of bowel movements, as well as some incontinence to flatus and stool. An alternative to the straight-tube CAA is creation of a colonic J pouch. The pouch is created by folding a loop of colon on itself in the shape of a J. A linear stapling or cutting device is inserted into the apex of the J, and the stapler creates an outer staple line while dividing the inner septum. The J-pouch anal anastomosis can be stapled or hand sewn.
An alternative to doing the entire dissection from within the abdomen is to begin the operation with the patient in the prone jackknife position. The perineal portion of this procedure involves an intersphincteric dissection via the anus up to the level of the levator ani muscles. After the perineal portion is complete, the patient is turned to the modified lithotomy position and the abdominal portion is performed. Either a straight-tube or colonic J-pouch anal anastomosis can be created; however, both must be hand sewn [26].
The advantages of the J pouch include decreased frequency and urgency of bowel movements because of the increased capacity of the pouch. A temporary diverting stoma is performed routinely with any coloanal anastomosis.
13.12.3 Abdominal Perineal Resection (APR)
APR is performed in patients with lower-third rectal cancers. APR should be performed in patients in whom negative margin resection will result in loss of anal sphincter function. This includes patients with involvement of the sphincters, preexisting significant sphincter dysfunction, or pelvic fixation, and sometimes is a matter of patient preference.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





