Carlos Abath • Romero Marques • Marcos Barbosa de Souza Júnior
Primary renal artery aneurysms (RAAs) are relatively rare, contributing to 1% of all aneurysms and 15% to 22% of visceral aneurysms.1 They have an estimated incidence of 0.09% in the general population, 0.1% to 2.5% in angiographic series, and up to 9.7% in autopsy series.2–4 However, the trend for more widespread investigation of the renal arteries with noninvasive methods has resulted in an increasing number of cases receiving medical attention.5
In most cases, the clinical relevance of the aneurysm is uncertain as the patient has no symptoms directly related to the aneurysm. Some patients may present arterial hypertension, renal ischemia, hematuria, or flank pain, but the cause-and-effect relationship is hardly established.4,6,7 The natural history of RAA is poorly documented. Although rupture is rare, with an incidence reported at 5% to 10%, it may be associated with mortality rates as high as 80%. The risk of RAA rupture is significantly increased in pregnancy and polyarteritis nodosa (PAN) and is also related to the aneurysm size.4,8–10
The accepted indications for RAA treatment include symptomatic patients, women who are pregnant or in those contemplating pregnancy, PAN, and enlarging lesions. Currently, no consensus exists for the size at which an RAA should be repaired in an asymptomatic patient. It is recommended that the lesion should be treated when greater than 1.5 to 3 cm, although most use 2 cm as the reference size for treatment.4,6,7,10,11
The surgical management of RAA is relatively safe, with a low mortality rate ranging from 0% to 4%. Otherwise, in complex lesions, intentional nephrectomy occurs in up to 20% of cases and unplanned nephrectomy in 5% of cases.1,6 With the progress of endovascular techniques and development of new devices, even very complex lesions can be selectively treated, sparing the normal vascular tree. Therefore, this less invasive procedure carries a much lower morbidity and mortality rate, assuring that a complete understanding of aneurysm angioarchitecture is achieved and a proper endovascular strategy is planned. In this chapter, we will describe our endovascular management of RAA, discussing the patient and endovascular technique selection criteria and determining the immediate and long-term anatomic results.
TECHNIQUE
Atherosclerosis and fibrous dysplasia are the most common causes of aneurysm formation. This can also be associated with some systemic diseases, such as PAN, neurofibromatosis, and tuberous sclerosis. Traumatic and iatrogenic renal artery pseudoaneurysms are frequent vascular lesions but represent another pathophysiologic entity.
Over the last decade, we have treated 21 cases of nontraumatic RAA. There were 16 females and 5 males, with a mean age of 51.2 years (range, 19 to 79 years). Six patients had hypertension, 2 of them with a solitary kidney. Five other patients presented flank pain related to the side where the aneurysms were located. Two other patients had hematuria. In the 8 remaining patients, the aneurysms were found incidentally.
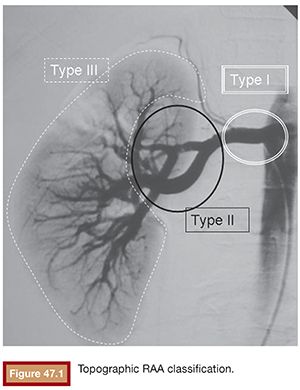
Atherosclerosis, fibromuscular dysplasia, and tuberous sclerosis coexisted in 4, 14, and 3 patients, respectively. The mean aneurysm diameter was 26 mm (range, 15 to 38 mm). For the purposes of endovascular treatment strategy, the RAAs were classified according to their location12,13 as type I, main renal artery (3 cases); type II, arterial bifurcations (14 cases); and type III, distal intrarenal (3 cases) (Fig. 47.1).
The endovascular treatment of RAA must be precise, safe, and durable, achieving complete aneurysm exclusion from the blood circulation and preserving, as much as possible, the arterial tree and renal parenchyma. To achieve these goals, the lesions can be treated by stent graft implant or selective embolization of the aneurysm sac. Unfortunately, both of these techniques have limitations and cannot be applied to every case. It is sometimes necessary to perform a parent vessel occlusion to treat the aneurysm, with some grade of renal parenchyma compromise. A full understanding of the arterial anatomy is needed for the recognition of challenging technical difficulties that will guide the choice of the best strategy and proper tools for each specific case. Complex and large lesions, broad-necked aneurysms, and bifurcation-located aneurysms, with arterial branch involvement, must be well identified and studied before the endovascular treatment. The noninvasive diagnostic methods, mainly the multidetector computed tomography, can provide valuable information regarding the aneurysm size, mural thrombus, wall calcification, and relationship with the parent vessel. Digital angiography with three-dimensional (3-D) reconstruction remains the best method for a pretreatment anatomic evaluation.
Angiography
As usual, the diagnostic angiography, via a femoral approach, begins with an aortography, unless there is an abnormal renal function. This initial study may show additional aneurysms or other lesions in the contralateral kidney, which can change the original treatment plan. It also can show the presence of accessory renal arteries. A selective renal angiography is then performed, in anteroposterior and oblique views, to demonstrate the aneurysm neck on profile. The distances from the aneurysm to renal artery ostium and renal artery bifurcation must be shown and are essential to the decision of placing a stent graft. The involvement of arterial branches by the aneurysm must also be disclosed. If the aneurysm is large or close to the renal bifurcation, it can be extremely difficult to obtain adequate visualization of the structures because the aneurysm sac is superposed over the renal vascular tree. Therefore, it is very useful to do a rotational angiography for 3-D image reconstruction. With the aid of a workstation, it is possible to play with the 3-D images to get the best views and to find the ideal working projection, where the neck is visualized on true profile. Once the working projection is found, the arch is positioned according to the angulations displayed in the workstation. Another advantage is the possibility of obtaining the vessel and aneurysm measurements with a minimal error margin. In our practice, we use Allura (Philips Healthcare, The Netherlands) or Artis zeego (Siemens Corporation, Malvern, Pennsylvania) equipment, doing the rotational angiography with 20 mL of nonionic contrast at a flow rate of 4 mL per second.
Stent Graft
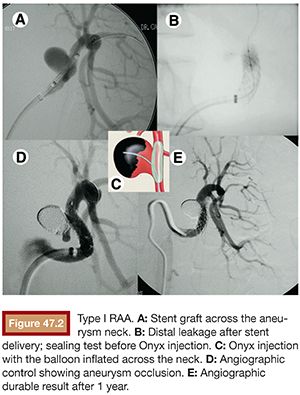
Despite the development of new, more flexible, low-profile devices, the stent graft has restricted use in the treatment of RAA. It should not be applied in type II aneurysms because it would cover an important branch at the bifurcation, leading to a major renal infarction. It is not useful in type III distal lesions either because it does not have enough flexibility and low profile to navigate in tiny and tortuous vessels. The stent graft is a treatment option only in selected type I aneurysms, when the main renal artery is straight and the edges of the aneurysm neck are located at least 15 mm away from the renal ostium and hilar bifurcation. If these limits are not respected, there is a risk of technical failure, resulting in endoleak and persistent flow inside the aneurysm pouch. This occurred in the only case in which we tried to use a stent graft to seal a type I aneurysm, and the procedure had to be complemented by Onyx (Covidien, Irvine, California) embolization (Fig. 47.2).
Thromboembolism is another problem related to the use of stent graft in small-sized vessels such as the renal arteries, demanding a long-term administration of double antiplatelet therapy with acetylsalicylic acid and clopidogrel.
Flow-Diverter Stents
Flow-diverters stents have been developed for endovascular treatment of intracranial aneurysms, and today, two such stents are available: the Pipeline (Covidien, Plymouth, Minnesota) stent and the Silk stent (Balt Extrusion, Montmorency, France). Although they represent a safe, durable, and curative treatment of selected wide-necked, large, and giant intracranial aneurysms, important complications such as stent thrombosis, side branch occlusion, or postimplantation bleeding have been reported.14
The Cardiatis multilayer stent (Cardiatis, Isnes, Belgium) is a new type of flow-diverter stent consisting of two interconnected layers without any coverage, leading to decreased turbulent flow velocity in the aneurysm sac while improving laminar flow in the main artery and its branches. The stent is made of a biocompatible cobalt alloy wire, is available in diameters from 2 to 50 mm, and can be loaded in small (6-Fr) delivery systems.15 Even though it is a promising tool for the endovascular treatment of some selected visceral and peripheral aneurysms, only few case reports and small series have been published.15 At the moment, this technology should be restricted to the rare cases where the other options are not applicable until its clinical effectiveness and safety is proved in further studies with longer follow-up.
Selective Coil Embolization
The advent of microcatheter and guidewire systems and new embolic agents allowed a selective occlusion of the most RAAs regardless of the topographic localization. The best lesions for this endovascular approach are saccular aneurysms with a well-defined narrow neck, measuring less than 4 mm or presenting a dome-to-neck proportion equal or superior to 2:1, without arterial branches arising from the aneurysm.
Preference is given to perform the procedure under general anesthesia for controlled apnea and generous use of road mapping. The embolization is safer and quicker and contrast volume is reduced. After digital subtraction aortography, selective renal artery catheterization is done with a double curve guiding catheter 6-Fr (Mach 1, Boston Scientific Corporation, Natick, Massachusetts). Thereafter, superselective catheterization of the aneurysm is performed with the Excelsior 14 microcatheter (Boston Scientific Corporation, Natick, Massachusetts) over a platinum tip 0.0014-in steerable guidewire (Transcend; Boston Scientific Corporation, Natick, Massachusetts) under road mapping fluoroscopy. The microcatheter is advanced coaxially through the guiding catheter, which is fitted proximally with a rotating hemostatic valve. The coaxial system is continuously flushed with heparinized saline solution to prevent thrombus formation in between the catheters.
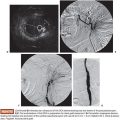
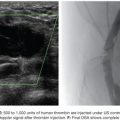
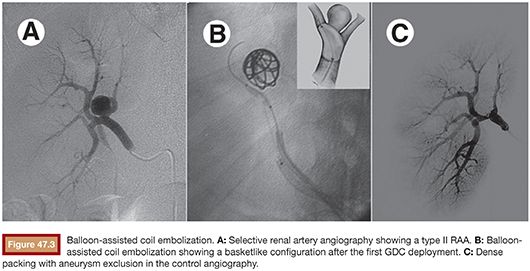
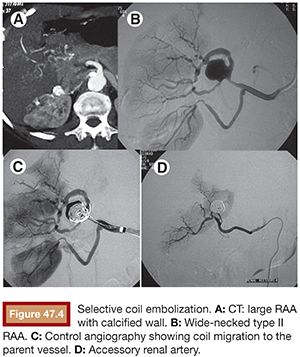
Once the microcatheter tip is inside the aneurysm, the cavity is progressively filled with multiple detachable microcoils. Currently, there are many types of detachable microcoils in the market from different manufacturers. In most of our cases, the Guglielmi detachable coil (GDC; Boston Scientific Corporation, Natick, Massachusetts) is used. GDCs are circular or multishaped soft platinum coils, varying in size and length. The coil is attached to a Teflon-coated stainless steel delivery wire by a short portion of uninsulated stainless steel. After the correct position of the implanted coil is confirmed angiographically, a positive direct electrical current is applied to the proximal end of the stainless delivery wire. The negative pole is connected to a needle positioned in the patient’s skin. Within 1 minute, the current dissolves the uninsulated stainless steel section proximal to the platinum coil by electrolysis and the delivery wire is then withdrawn. The size of the first coil should equal the aneurysm size, achieving a basketlike configuration within the sac (Fig. 47.3B). The remaining cavity is filled with smaller coils, which are placed within the network of the first GDC, until the whole cavity of the aneurysm is densely packed with coils (Fig. 47.3C). Unfortunately, this technique alone cannot be applied to more complex lesions, such as large-neck aneurysms or when arterial branches arise from the aneurysm sac. In these cases, it is essential to perform associated remodeling techniques to protect the neck and avoid parent vessel or branch occlusion. This complication was observed early in our experience, when we did not protect the wide aneurysm neck in a type II lesion (Fig. 47.4).
REMODELING TECHNIQUES
Until the development of remodeling techniques, wide-necked aneurysms could not be treated by selective embolization without a significant risk of parent vessel occlusion caused by coil migration into the unprotected parent artery. In the remodeling technique, a temporary balloon is inflated intermittently at the aneurysmal neck while the microcoil or other embolic agents are deposited. Alternatively, a stent can be delivered to protect the neck, acting as a scaffold in the parent artery, while coils or other embolic materials are deposited into the aneurysmal lumen.
Balloon-Assisted Coiling
The balloon remodeling technique, first described by Moret and coworkers16 in 1997, was the initial alternative to surmount the problem of wide-necked brain aneurysms. In this technique, a soft semicompliant or conformable balloon is positioned across the neck of an aneurysm and inflated during coiling. The balloon works as a mechanical barrier that allows tighter packing of the aneurysm while preventing coil herniation into the parent artery during coil delivery. Also, the balloon stabilizes the microcatheter during coil delivery and forces the coils to conform to the 3-D shape of the aneurysm. The best balloons, specially designed for this technique, are the flexible, very low profile, compliant balloons: HyperGlide and HyperForm (Covidien, Plymouth, Minnesota). We routinely use the HyperGlide, leaving the HyperForm for some aneurysms located in bifurcations. Unfortunately, in some cases, the size of these balloons, which reaches only 4 mm in diameter, does not fit the main renal artery diameter, which ranges from 5 to 7 mm. So, they must be replaced by regular peripheral balloons with the desired diameters (Aviator plus; Cordis Corporation, Somerville, New Jersey).
When the balloon remodeling technique is performed, it is necessary to use a larger 7-Fr guiding catheter (Mach 1, Boston Scientific Corporation, Natick, Massachusetts) or a 6-Fr renal sheath introducer (Flexor® Cook Medical, Inc., Bloomington, Indiana), allowing passage for a balloon and a microcatheter at same time. Alternatively, we can do bilateral femoral puncture, introducing one 6-Fr guiding catheter in each side for the balloon and microcatheter, respectively. Special attention must be paid to the heparinization, keeping the activated coagulation time (ACT) three times the basal level. With the guiding catheter selectively placed in the main renal artery, the deflated balloon is positioned in the parent vessel, across the aneurysm neck. Selective microcatheterization of the aneurysm is then performed. Inflation of the balloon across the aneurysm neck temporarily occludes the neck and the parent vessel. Under balloon protection, coils are then delivered into the aneurysm. After placement of each coil into the aneurysm but before detachment, the balloon is deflated to test the stability of the coil. If no displacement of the coil is observed, the coil is detached. If movement is detected after balloon deflation, the coil is considered unstable and repositioned or removed. The procedure is repeated to obtain a dense and stable packing (Fig. 47.3).
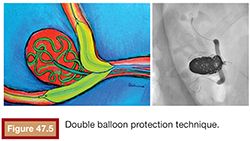
Sometimes, when both branches are involved by a large-neck aneurysm located at the bifurcation, a double balloon protection technique is required to avoid occlusion of one of the branches by coil herniation (Fig. 47.5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree