, Marc H. Schiffman1 and Thomas A. Sos1
(1)
Division of Interventional Radiology, Department of Radiology, New York Presbyterian Hospital/Weill Cornell Medical College, New York, NY, USA
Keywords
Renal artery stenosisRenovascular hypertensionAtherosclerosisFibromuscular dysplasiaRenal artery angioplastyRenal artery stentingCORALEmbolic protection devicesRenal artery stenosis, resulting in renovascular hypertension, is a major cause of secondary hypertension [1]. When both kidneys are affected, ischemic nephropathy with diminished renal function also results. The most common cause of renal artery stenosis is atherosclerotic, usually ostial, narrowing of the renal artery. The second most common cause of renal artery stenosis is fibromuscular dysplasia. In most cases, aRAS is treated with stent placement and FMD with angioplasty alone. Nonrandomized single-center series for revascularization for both atherosclerotic Renal Artery Stenosis (aRAS) and fibromuscular dysplasia (FMD) have reported excellent outcomes [2, 3]. In recent years, however, large prospective randomized trials have disputed these previously reported outcomes in the management of aRAS [4, 5]. Despite many flaws in these trials, the importance of proper patient selection and performance of appropriate endovascular technique by skilled operators has been emphasized. In this chapter, the authors review patient selection factors, describe standard techniques for renal revascularization, and provide technical “tips” that can be utilized during intervention. In addition, the authors will review reported outcomes, in particular, from several large retrospective studies including the primarily US-based “Cardiovascular Outcomes in Renal Atherosclerotic Lesions” (CORAL) trial.
Renal artery stenosis (RAS) refers to a fixed narrowing of the renal artery which can lead to renin-mediated renovascular hypertension (RVH) and ischemic nephropathy (IN). It is the most common cause of secondary hypertension and accounts for 1–4 % of all patients with hypertension [6].
Renal artery stenosis secondary to atherosclerosis (aRAS) accounts for 90 % of patients with RVH, with fibromuscular dysplasia (FMD) accounting for the second most common cause [6]. Angiographically, most aRAS lesions occur within the ostium or the first centimeter of the renal artery and are due to plaque within the aorta engulfing the ostium of the renal artery resulting in the stenosis [3]. The narrowing within FMD usually occurs in the mid to distal third of the renal artery and medial fibroplasia, with a “string of beads” appearance, which accounts for most cases [4].
Indications for treatment with renal artery catheter-based interventions must include renal artery stenosis documented to be hemodynamically significant, particularly when associated with renal dysfunction, poorly controlled hypertension, or recurrent pulmonary edema [7]. Renal revascularization certainly has a significant role in the management of aRAS patients; however, careful selection is necessary to identify patients who will benefit the most from intervention. Treatment for aRAS has been renal revascularization with renal artery angioplasty and stenting; however, this has been controversial secondary to several large-scale retrospective studies in recent years [4, 5]. Treatment for FMD with renal angioplasty has been shown to be widely accepted and efficacious.
Techniques
Patient Selection
Appropriate patient selection for intervention in patients with significant renal artery stenosis is based on clinical, anatomic, and physiologic (hemodynamic) criteria. Renal artery stenosis can be an independent clinically asymptomatic condition, or it can overlap with multiple clinical syndromes including hypertension or renal insufficiency [8]. All three clinical conditions can occur independently or may be causally linked to each other (Fig. 23.1). For example, patients with essential hypertension may have renal artery stenosis, which does not result in renovascular hypertension. It is important to stratify these separate clinical conditions in order to guide safe, effective, and appropriate treatment.
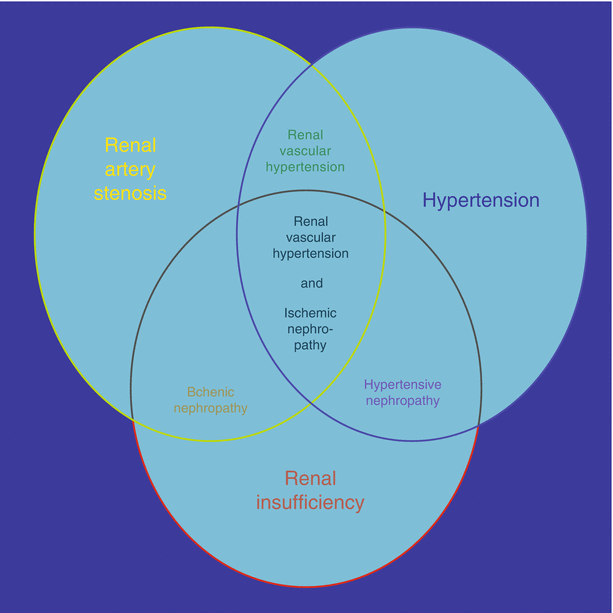

Fig. 23.1
Complex overlapping relationship between renal artery stenosis, hypertension, and renal insufficiency
Clinical Criteria
According to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment for High Blood Pressure, testing for identifiable causes of nonessential hypertension is not indicated unless blood pressure control is not achieved despite attempt with optimal doses of three drugs of different classes [9]. The Society of Interventional Radiology and American Heart Association states that patients should undergo further testing with imaging in the following group of patients: patients with onset of hypertension before 30 years of age; patients with severe hypertension after the age of 55; accelerated, resistant, or malignant hypertension; new azotemia; worsening renal function after administration of angiotensin converting enzyme inhibiting agents or angiotensin receptor blocking agents; size discrepancy between kidneys greater than 1.5 cm or sudden; and unexplained pulmonary edema [10, 11]. Physical exam and imaging findings in patients with suspected RAS may include continuous epigastric bruit, retinopathy, and asymmetric renal atrophy. Laboratory results, which should prompt further investigation with diagnostic imaging or angiography, include elevated plasma renin activity, hypokalemia, and/or proteinuria [11].
Anatomic Criteria/Screening Diagnostic Exams
In patients with suspected renovascular hypertension, imaging technique selection for diagnostic screening should be guided and based on the American College of Radiology Appropriateness Selection Criteria [12]. These include ultrasound, nuclear medicine exam, computed tomography (CT), and magnetic resonance angiography (MRA). CT and MRA have the added benefit of not just being screening exams but can be vital in the pre-procedural planning prior to angiography.
Duplex Doppler ultrasound (US) has the highest rating in appropriateness criteria for the evaluation of patients with high index of suspicion of renovascular hypertension and diminished renal function and second highest in patients with normal renal function [12]. US provides real-time anatomic and hemodynamic information by utilizing gray scale, color flow Doppler, and Doppler spectral analysis to evaluate the renal arteries. A diagnosis of RAS on ultrasound requires a peak systolic velocity in the renal artery exceeding 180 or 200 cm/s and a renal artery/aortic velocity ratio exceeding 3.5. Doppler US can also be helpful in predicting outcomes for renal artery interventions. Resistive indices of greater than 0.8 have been shown to predict poor outcome after attempts at reducing hypertension and improving renal function [13], but others dispute its predictive value. Unfortunately, US is operator dependent and also may be compromised by the patient’s body habitus. These limitations may become significant when patients are serially followed and the exam is performed by different operators.
Nuclear medicine screening exams offer the added advantage of providing functional renal assessment along with limited anatomic information. Renal perfusion, renal function, and renal excretion can be qualitatively and quantitatively assessed. For the diagnosis of renal artery stenosis, the study of choice is angiotensin converting enzyme inhibitor scintigraphy. Kidneys affected by renovascular hypertension demonstrate impaired function due to angiotensin converter enzyme inhibition. The reported sensitivity of ACE-inhibitor renal scintigraphy for renovascular hypertension ranges from 34 to 93 % and with specificity of 100 % [14].
Computed tomography angiography (CTA) uses multi-detector row helical scanners to generate high-resolution two-dimensional images and volume or surface renderings in three dimensions to provide detailed anatomic information [15]. According to the ACR Appropriateness Criteria, CTA is rated the highest in patients with high index of suspicion of renovascular hypertension and normal renal function [12]. Along with detailed anatomic information, additional information about the renal arteries that CTA can provide includes the extent and distribution of atherosclerotic plaque as well as secondary signs of RAS including post-stenotic dilatation, changes in renal size and volume, and cortical changes [16]. Although CTA can be useful in evaluating renal artery stents and in stent stenosis, imaging artifact from high-density materials including calcified arterial plaques can cause beam hardening and limit evaluation of surrounding structures. A major limitation of CTA besides the use of significant doses of ionizing radiation is the use of nephrotoxic iodinated contrast – alas in patients who need it most, those with IN, it often cannot be used.
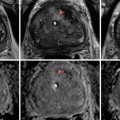
Along with CTA, magnetic resonance angiography (MRA) is a highest rated exam of choice according to the ACR Appropriateness Criteria in patients with a high index of renovascular hypertension and normal renal function (Fig. 23.2). MRA is also the highest rated exam of choice in patients with diminished renal function given the ability to obtain information without the use of an iodine-based contrast agent [12]. Gadolinium-based MRA contrast unfortunately has been associated with nephrogenic systemic fibrosis (NSF) in patients with IN. Newer noncontrast MRA sequences with steady-state free precession (SSFP) and arterial spin labeling techniques and safer Gad-based contrast can overcome these problems. MRA uses magnetic fields and radiofrequency energy to produce high detailed images, which provide information in identifying RAS, vascular anatomy, renal anatomy, and technique planning. The negative predictive value of unenhanced MRA compared to CTA and DSA is 96 % suggesting negative MRAs essentially nearly rule out renal artery stenosis [17, 18]. Newer techniques, such as blood oxygen level-dependent (BOLD) MRI, are being developed to provide physiologic information that may help identify patients who will benefit from the intervention [19–22].
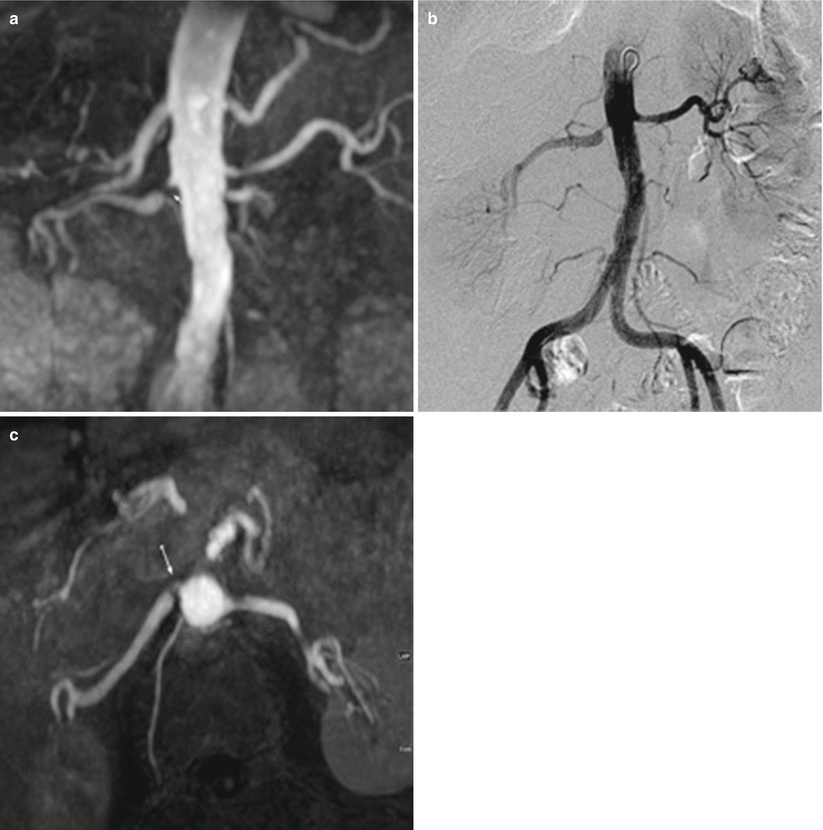

Fig. 23.2
Patient presenting with symptoms of renovascular hypertension with suspected right-sided renal artery stenosis on pre-procedural MRA (a) and confirmed on angiography (b) after pressure measurements obtained. Axial slice from MRA (c) demonstrates ostial stenosis of the right renal artery, which arises approximately 30° ventrally along the right anterolateral aspect
Physiologic Criteria: Catheter-Based Angiography with Pressure Measurements
Catheter-based angiography is the gold standard for diagnosing significant renal artery stenosis [23]. Catheter arteriography has high spatial resolution for evaluating the main renal arteries as well as branch renal arteries. The anatomic minimum threshold of hemodynamically significant renal artery stenosis is greater than 70–80 % diameter [24, 25]. Visual estimation of a stenosis based on angiography is notoriously inaccurate and must be confirmed by physiological/hemodynamic objective assessment with pressure gradients across a stenosis. Hemodynamically significant stenoses include lesions with a peak systolic gradient of greater than 10–20 mmHg or 10 % of the peak systolic pressure [26–28].
Multiple studies have proven that pressure gradients are the most effective and reliable indicator for how well a patient will respond to angioplasty and stenting. Leesar et al. in 2009 and Mangiacapra et al. in 2010 compared systolic pressure gradients, renal fractional flow reserve, quantitative angiography, and intravascular ultrasound and found that systolic pressure gradients had the best receiver operator characteristic curve for predicting blood pressure response post renal artery stenting [26, 28].
Renal Vein Renin Sampling
Although the usefulness has been controversial, renal vein renin (RVR) sampling can be an additional physiological indicator of how much a renal artery stenosis is contributing to a patient’s hypertension. In addition, it can be a useful predictor in determining which patients will benefit the most after revascularization for renovascular hypertension [7]. Studies have shown, though, that RVR is neither sensitive nor specific enough to exclude patients who do not have RVH [29–32]. RVR secretion that lateralizes to the affected side, however, has as strong positive predictive value [33].
Venous samples should be taken from the right main renal vein, from the left renal vein, and from the infrarenal IVC. The renin levels can be analyzed by the simple ratio method or the incremental ratio method. The simple ratio method takes the ratio of the RVR activity in the involved size divided by the activity in the non-involved side with a positive ratio being 1.5:1.0 (sensitivity 62 % and specificity 60 %) [7]. The incremental ratio method calculates the net secretion of the renin from each kidney. Hypersecretion of renin from the stenotic kidney ([V − A]/A > 50 %) and contralateral suppression release from the normal kidney are strong predictors of curability of hypertension with therapeutic revascularization for the affected kidney [30].
Potential pitfalls in RVR sampling, which can result in poor predictive values, include sampling renal veins in patients who are chronically blocked with ACE inhibitors or beta-blockers. In addition, failure to identify normal anatomic variants or accessory renal veins and not sampling distal to the gonadal vein in the left renal vein can both lead to suboptimal studies [7].
Summary: Algorithms for Treatment
After clinical suspicion of renovascular hypertension or renal insufficiency is raised and a screening diagnostic exam is positive for renal artery stenosis, the anatomic and hemodynamic significance of stenosis must be confirmed by pressure gradient, and the patients should be treated. Finally, safe and reasonable anatomic and medical conditions for access and therapy should be present. A summary of the algorithm for workup and management in patients with suspected renovascular hypertension and renal insufficiency is shown in Fig. 23.3.
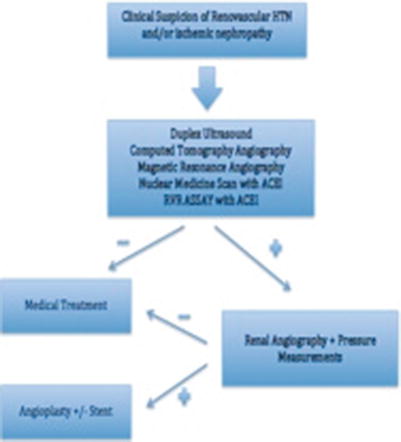

Fig. 23.3
Algorithm for the management of patients with renovascular hypertension secondary to suspected renal artery stenosis
Absolute contraindications to treatment include a hemodynamically insignificant stenosis and a medically severely unstable patient. Relative contraindications include a long-segment occlusion, an atrophic kidney, and a severely diseased aorta predisposing to increased risk of embolization of atheroma. Newer catheters and techniques performed by skilled operators, however, allow interventionalists to proceed despite these relative contraindications [7] (Fig. 23.4).
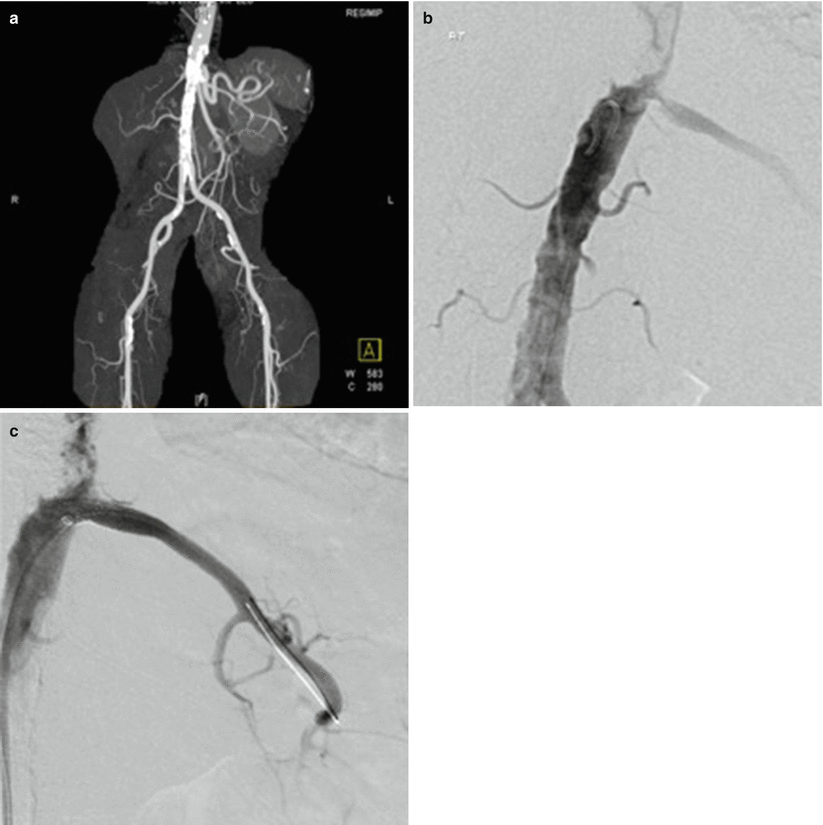

Fig. 23.4
An 82-year-old female with refractory hypertension and imaging findings of left renal artery stenosis, right renal artery occlusion, and severe atheromatous aorta (a). Angiography and pressure measurements confirmed left renal artery stenosis with a trans-stenotic mean arterial pressure gradient of 22 mmHg and severe suprarenal aortic plaque (b). Successful stent placement across left renal artery stenosis (c)
Diagnostic Catheter-Based Aortography
Initial puncture is usually made through the common femoral artery. Interventional cardiologists are increasingly using the radial artery, and vascular surgeons are increasingly using the brachial artery [34]. Specific anatomic considerations, which may affect the puncture selection, have to be taken into account during pre-procedure planning and include the obliquity of the renal arteries at their origin from the aorta (see section “Tips”).
After puncture, a standard 10 cm 5 Fr vascular sheath is placed, and a flush catheter, such as a 4 Fr OmniflushTM (Angiodynamics, Queensbury, NY) catheter, is used for standard aortography. The advantage of using the OmniFlushTM catheter is its ability to deliver a more concentrated bolus to the area of interest because its side holes and end hole are directed laterally and caudally [7]. The catheter is positioned at the T12–L1 interspace since the renal arteries usually originate at the upper endplate of the L1 vertebral body. After aortography, the renal artery should be selected with a soft atraumatic wire, such as a Bentson wire, and a recurved catheter such as the Sos OmniTM selective (Angiodynamics, Queensbury, NY). Hydrophilic guidewires should be avoided as much as possible given their propensity to result in vascular perforation; however, if they are used to cross difficult stenosis, whenever possible, they should be exchanged for a non-hydrophilic wire. Multiple visceral recurved diagnostic catheters can be used for selecting the renal arteries including a Simmons, Sidewinder, Shepherd’s crook, and Sos Omni SelectiveTm or the nonrecurve Cobra Tips for selecting the renal artery is discussed in greater detail below within the section “Tips.”
Pressure Gradient Measurements
After successfully crossing a lesion with a soft atraumatic wire and a recurved catheter, the transtenotic pressure gradient should be measured, ideally with a 0.014″ pressure-sensing wire [35]. This translesional gradient should then be compared to a simultaneous aortic pressure measured, ideally, with a multichannel monitor. Serial alternating pressures can be compared if a multichannel monitor is not available. If a pressure-sensing wire is not available, pressure measurements can be obtained through a 4 Fr catheter; however, it should be noted, and this catheter contributes to the existing stenosis [7]. Since visual assessment of anatomic stenosis is very unreliable, even in seemingly very severe stenosis, pressure gradients must always be measured.
Angioplasty
Percutaneous transluminal renal angioplasty (PRTA) is a nonsurgical method of restoring blood flow within RAS. Balloons vary tremendously in length, diameter, shape, compliance, and material. Choosing a balloon depends on several factors, which include the length and severity of stenosis, diameter of the non-stenotic portion of the renal artery, tortuosity, and branching pattern. PTRA alone has been found to be more successful in non-ostial (1 cm from the origin) lesions and is indicated in stenosis due to either atherosclerosis or fibromuscular dysplasia. In aRAS ostial lesions, PTRA alone fails in up 75 % of cases [36]; primary stenting is the preferred treatment. Complications from PTRA and stenting include distal embolization, cholesterol embolization, renal artery injury, and access site hematoma [7].
Initially diagnostic aortography and selective renal artery catheterization with trans-stenotic pressure measurements are performed, as previously described. When PTRA is planned, careful selection of the size of the balloon diameter should be made. Generally, a balloon diameter that is 10 % larger than the estimated “normal” diameter of the vessel based on the arteriogram is recommended. Sub 4 Fr balloon systems are used with 0.014″–0.018″ guidewires or a 5 Fr balloon system over a 0.035″ guidewire if necessary [7]. A soft-tipped but relatively stiff shaft wire is used to select and cross the stenosis, and patients are systemically heparinized once the lesions are crossed. The diagnostic catheter used to cross the stenosis is then exchanged for a longer sheath/guide system or directly for the balloon catheter; alternatively, the sheath can be introduced initially and all maneuvers performed through it. After placing the balloon markers across the lesion, the balloon is slowly inflated until the balloon is fully inflated or has reached its rated maximum pressure. In long-segment FMD, overlapping inflations may be necessary, starting distally and proceed centrally. After removing the balloon catheter, a “post-” PTRA angiogram is performed, ideally utilizing a technique that preserves wire position across the lesion. In addition a post-PTRA pressure gradient is performed to ensure that the gradient has been resolved. This is the strongest indicator for posttreatment success rates [28].
Stenting
Renal artery stenting improves the efficacy of PTRA by offsetting the recoil force of arteries and atherosclerotic plaque, especially at the ostium [7]. Almost all aRAS stenoses at the ostium require a stent. After diagnostic angiography, the renal artery and stenosis are crossed, as described previously in the renal angioplasty section (Fig. 23.5). Multiple techniques exist for proper stent deployment, in particular for renal ostial lesions. With renal artery stenting, a guiding catheter/sheath system is almost always used for stent deployment. The sheath or guiding catheter typically acts as support or “backbone” to help with crossing tight renal artery stenosis. In addition, it allows for contrast injections, while equipment including balloons, wires, and stents are in place [37]. A stent and dilating balloon, which will adequately cover the lesion, extend a few millimeters into the aorta and match the normal vessel diameter that is used.
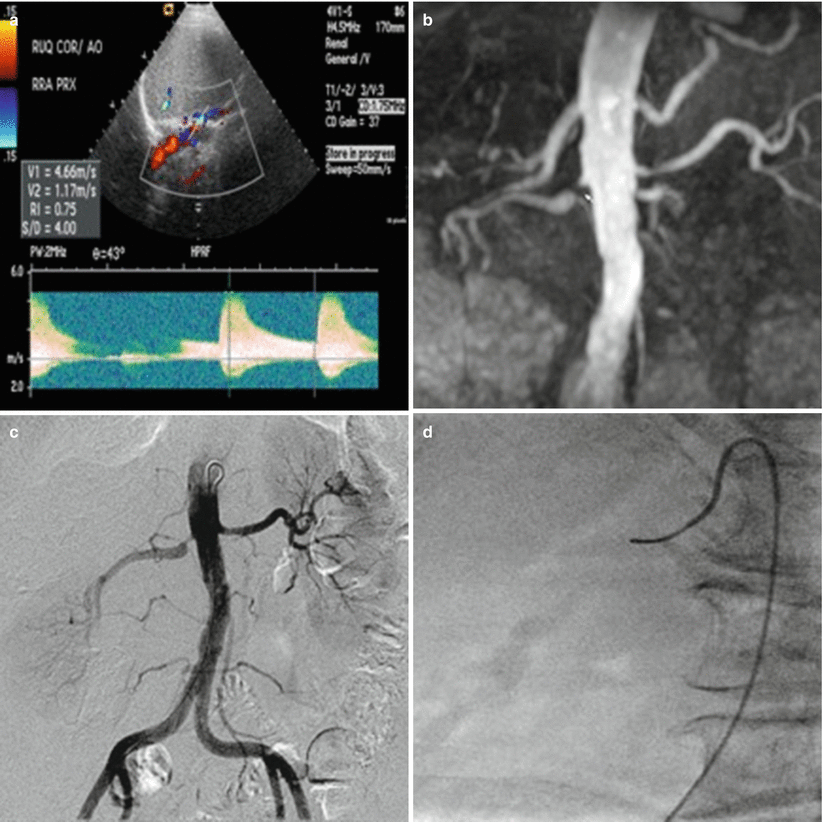
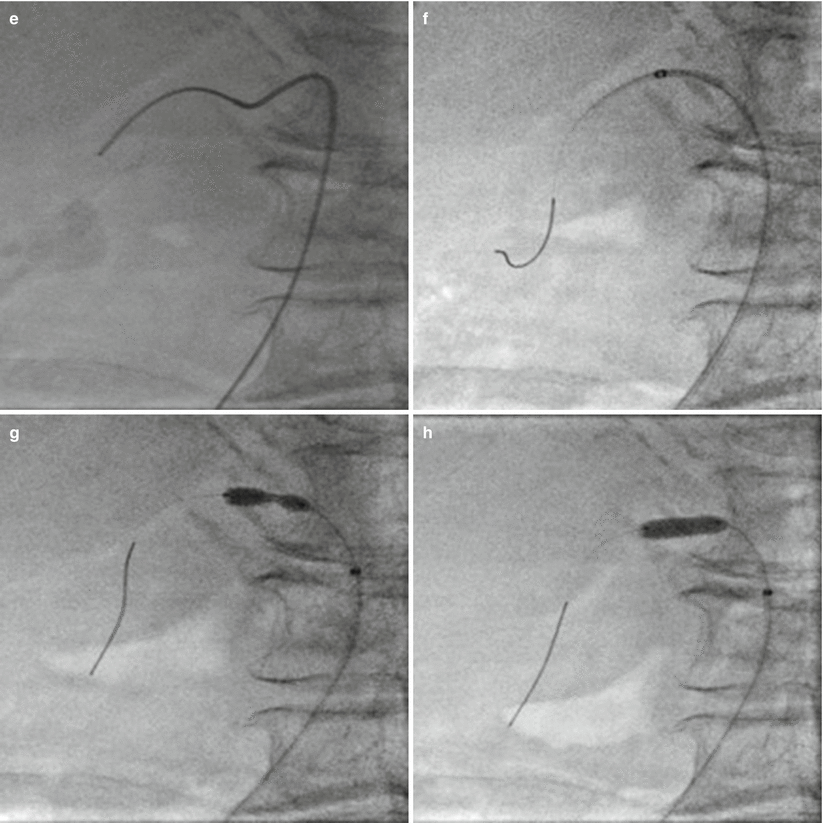
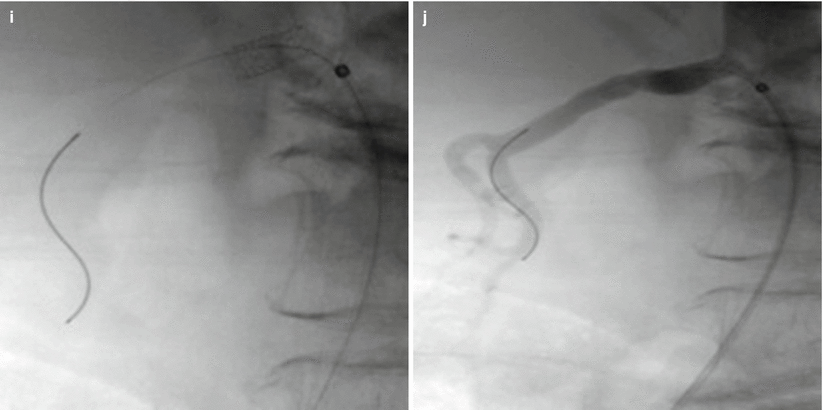



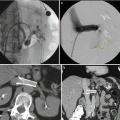
Fig. 23.5
A 77-year-old female with refractory hypertension on ACEI and two additional antihypertensives with elevated plasma renin activity. (a) Ultrasound demonstrates elevated peak systolic velocity and elevated arterial resistive indices suggestive of renal artery stenosis. (b) MRA demonstrates severe stenosis at origin of right renal artery with post-stenotic dilatation. (c–j) A 4 French Sos Omni 2 catheter and a Bentson wire were used to select the right renal artery. Pressure measurements demonstrated a 49 mmHg mean arterial trans-stenotic pressure gradient. The Bentson was exchanged for a 0.018″ Ironman, and the Sos Omni 2 Catheter and sheath were exchanged for a long 5 French Ansel Sheath. A 5 mm × 15 mm Express Tm SD Renal and Biliary Pre-mounted (Monorail) Stent System (Boston Scientific, Marlborough, MA) was successfully placed in the proximal right renal artery
A 0.018 or 0.014 in. atraumatic wire and a diagnostic catheter are utilized to cross the lesion, and the wire tip is positioned in the distal main renal artery or a proximal first or second order branch. The stent is pre-mounted on a balloon catheter and is delivered through a 5 Fr or 6 Fr guide sheath or catheter. In cases of severe stenosis, the 5 Fr sheath may be advanced through the stenosis to ascertain stent passage and then slowly withdrawn leaving the balloon/stent combination over the guidewire in the stenosis [38]. For low-profile pre-mounted Monorail balloon-expandable stents, after a stiff guidewire is placed across a lesion, the stent is positioned across the lesion with about 2 mm extending into the aorta. Accurate positioning is confirmed by injecting contrast through the sidearm of the sheath, the balloon is then inflated, and the stent is deployed. To achieve long-term patency, it is mandatory for the stent to cover the stenosis extending from the ostium into the aorta and a few mm distal to the stenosis. Completion arteriography is performed through injection of the sidearm of the sheath after it is withdrawn into the proximal stent or aorta. Overlapping stents reduce patency; if they are necessary, approximately 2 mm of stent overlap is recommended. Gaps within a series of stents may promote intimal hyperplasia. Once completed, repeated angiograms through the sheath and pressure measurements should be taken to evaluate positioning, residual stenosis, or residual abnormal pressure gradients.
Specific Situations
Bilateral Renal Artery Stenosis and/or Occluded Renal Artery
Treatment of bilateral renal artery stenosis should be performed during the same session, if possible. If both sides have lesions thought to be hemodynamically significant, the technically easier side, which is often that with the larger kidney, should be attempted first; if the first procedure is successful and both the operator and patient are still fresh, the second more difficult side can be attempted but rescheduled or postponed if significant difficulties are encountered.
If the technically more difficult and functionally less important side were attempted first and resulted in a complication or were unsuccessful, a tired operator and patient may have to perform/undergo the second “rescue” procedure which probably would have been successful if it had been performed initially, but now under adverse conditions, it is less likely to succeed. If for any reason, the technically more challenging cannot be treated, the patient has the benefit of at least one side being treated.
In cases of renal artery occlusion, recanalization should only be attempted if the patient is anatomically or physiologically uninephric or after the contralateral kidney is successfully treated. Criteria for intervention of a completely occluded renal artery include clinical significance and adequate kidney size, if a clear stump is seen on angiography and if there is increased renin production [39]. Pre-procedure planning with CTA or MRA will assist in determining the anatomy of the occlusion. A straight hydrophilic guidewire can be used to cross the occluded segment through a catheter which can pull/push the wire and catheter through the occlusion. Typically a stiff one piece 5 F AngiOptic Sos Omni™ or similar recurve selective catheter is used; however, great care should be taken given the significant risk of vessel or collecting system perforation with the stiffer hydrophilic guidewire [40]. As soon as the occlusion is crossed, a control arteriogram through the sheath must be performed to ascertain that the wire tip is in the distal lumen. If the recanalization resulted in extravascular passage, the procedure should be stopped and the vessel observed for bleeding for at least an hour and even embolized if necessary. If the recanalization was successful, the hydrophilic guidewire should be immediately exchanged with a non-hydrophilic soft platinum-tipped stiffer shaft wire and stenting continued.
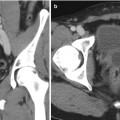
Transplant Renal Artery Stenosis
Transplant renal artery stenosis is the most frequent vascular complication in patients with post renal transplantation. The most common time period in which transplant renal artery stenosis presents is at between 3 months and 2 years [41]. Renal revascularization is the treatment after medication regimen has failed and there is suspicion of renal artery stenosis based on a screening exam such as duplex ultrasound and/or magnetic resonance angiogram (Fig. 23.6). In cases with recurrent stenoses or an ostial stenosis, such as at an end-to-side renal to iliac artery anastomosis, stenting is recommended. Angioplasty, alone, usually in an end-to-end renal to hypogastric artery anastomosis – a procedure now rarely performed – has a reported success rate of greater than 80 % [42]. Stent placement has also been reported to have high clinical success, including significant early improvement of hypertension and creatinine, with minimal complications [43]. Eighty-eight to one hundred percent clinical success rates have been reported based on clinical outcome such as hypertension control, reduction in antihypertensive drugs, and graft function and preservation [44, 45




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree