, Valery Kornienko2 and Igor Pronin2
(1)
N.N. Blockhin Russian Cancer Research Center, Moscow, Russia
(2)
N.N. Burdenko National Scientific and Practical Center for Neurosurgery, Moscow, Russia
Renal cell carcinoma (RCC) is the most common malignant tumor of the kidney, accounting for about 80–90% of kidney tumors in adults and about 2–3% of all new cancer cases (Mori et al. 1998). RCC occurs twice as likely in men as in women, with the highest incidence in the age of 50–70 years (Muscat 2000). Each year, 30,000 and 20,000 new cases of kidney cancer are reported in the USA and in the European Union, respectively (Kirkali et al. 2001). In Russia, RCC occupies one of the first places among all forms of cancer in terms of the incidence rate: the number of cases in recent years approaches 20 thousand per year. Mortality rates from this disease in Russia are also high, 8000–9000 per year, accounting for 2–3% of all deaths of patients with malignant tumors. The 5-year survival in patients is 80–90% and 5–10% with localized and disseminated RCC forms, respectively (Motzer et al. 2004).
According to modern concepts, RCC is a multifactorial disease. A variety of factors can cause its development: genetic, hormonal, chemical, immunological, radiation, etc. (Ljungberg et al. 2011). Smoking, fatty and fried food, obesity, uncontrolled use of analgesics, diuretics, and hormone drugs significantly increase the risk of the disease (Lindblad et al. 1995; Coughlin et al. 1997; Godley and Stinchcombe 1999; Muscat 2000; Steliarova-Foucher and Parkin 2011). Thus, according to Parkin (2011), 42% of cases of RCC are caused by smoking and overweight. Men and women account for 47 and 34% among the cases, respectively. Vegetable diet and the use of vitamins with an antioxidant complex reduce the risk. Chronic renal failure and regular hemodialysis, polycystic kidney, nephrosclerosis often developing in diabetes mellitus, hypertension, and chronic pyelonephritis can also cause the development of kidney cancer.
Data from several meta-analyses indicate an increased risk of RCC up to fourfold in relatives of patients with renal cell carcinoma (Pfaffenroth and Linchan 2008; Karami et al. 2010). According to Clague et al. (2009), 4% of all RCC cases are due to hereditary factors. A family anamnesis of prostate cancer, melanoma, thyroid cancer, bladder cancer, as well as non-Hodgkin’s lymphoma is associated with an increased risk of kidney cancer (Liu et al. 2011).
There are three forms of malignant kidney tumors: renal cell carcinoma, adenocarcinoma originating from the pelvis, and kidney sarcoma known as Wilms’ tumor. Renal cell carcinoma has been described by Grawitz in 1883. This type of tumor is called hypernephroma; however, today the term renal cell carcinoma is used. The tumor develops from the epithelium of the proximal renal tubules.
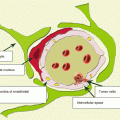
Multifocality of RCC, characterized by the simultaneous presence of more than one lesion with the same histological tumor type in the kidney, is one of its morphological features. Many authors noted RCC multifocality in their studies (Gohji et al. 1998; Baltaci et al. 2000; Lang et al. 2004; Richstone et al. 2004; Crispen et al. 2008). For example, in the group of cases studied by Shus (2003), the incidence of multifocal renal cell carcinoma was 16.7%.
Renal cell carcinoma has various types of the histological structure. Clear cell cancer is the most common, which, according to WHO, is 70–75% of all RCC cases. Men more often have typical RCC forms—clear cell and papillary cancer—while women more often have rare forms, mucinous, medullary, chromophobe, unclassifiable, and benign kidney tumors(Jemal et al. 2007; Yurin 2007; Davydov and Axel 2014a, b).
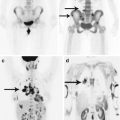
Malignant kidney tumors metastasize via the hematogenous and lymphogenous routes. Metastases occur more than in a half of patients. Often a kidney cancer metastasis manifests clinically earlier than the primary tumor, but in some RCC cases, metastasizing may not occur for decades (Radley et al. 1993). The most common sites of metastases are the lungs, lymph nodes, bone, liver, adrenal glands, brain, contralateral kidney, and heart.
Thus, a series of autopsies in patients with kidney adenocarcinoma showed brain involvement in 11% of cases (Saitoh 1981). According to Harada et al. (1999), among 325 patients treated for kidney cancer at the Osaka University Hospital from 1957 to 1993, 5.5% of cases were with brain metastases. Marshall et al. (1990) analyzed a series of cases of 106 patients with clinically localized renal adenocarcinoma, who underwent CT, and found metastases in the brain in 13% of cases. According to the authors, metastases to the brain most often occur in the later stages of kidney cancer, and the brain is likely to be the final site of the metastatic tumor. According to our data, kidney cancer metastases accounted for 13.9% of the total number of cases of metastatic brain lesions.
The interval between the detection of the primary kidney tumor and metastasizing to the brain ranges from 1 year to several years (Badalament et al. 1990; Nussbaum et al. 1996; Harada et al. 1999), and metastatic involvement of the meninges and skull bones in RCC occurs more frequently than in other histological forms of cancer. Multiple brain metastases of renal cancer are more typical for the young age (p < 0.001) (Bianchi et al. 2012). The most often metastasizing cancer is RCC (up to 50%), constituting 85% of all kidney cancers (Saitoh 1981).
Patients with renal cancer with distant metastases have a poor prognosis: their average life expectancy is 4.5–9 months. Kidney cancer patients with metastases to the brain have a poor prognosis as well: their average life expectancy is 5–9 months (Roser et al. 2002). Isolated cases of prolonged course of the disease with an identified kidney cancer metastasis were observed, which may be associated with low levels of mitosis, stability of the cell nucleus morphology, and, therefore, unexpressed chromosomal abnormalities. Treatment of these patients is complicated by the fact that kidney cancer belongs to radioresistant tumors, but radiosurgical treatment of metastases in the brain allows to reduce the tumor size. The effectiveness of chemotherapy in this form of cancer is low. Currently, the treatment of choice is surgery in combination with radiosurgery and immunotherapy(Patchell et al. 1990a, b; Coppic et al. 2000; Kleinberg 2009; Loudyi and Samlowski 2011; Nieder et al. 2011a, b; Blanco et al. 2011; Kusuda et al. 2011; Parashar et al. 2014; Raman and Vaena 2015).
A characteristic feature of intracranial metastatic RCC is intratumoral hemorrhages, often with the formation of an acute intracerebral hematoma requiring an urgent surgical intervention (Wronski et al. 1996). The author reports a series of cases of patients with renal cell carcinoma metastases, in which intratumoral hemorrhages were identified in 46% of cases and 4% of cases required emergency surgery. Our clinical cases are characterized by the relative homogeneity of renal cell carcinoma metastases: hemorrhages occurred not more than in 15% of cases.
Clinical symptoms of kidney cancer metastases in the brain are caused by a combination of cerebral and focal symptoms characteristic of metastases and primary tumors with other locations; it depends on the lesion site in the brain, size, and presence and severity of perifocal edema: headache, hemiparesis, cognitive impairment, seizures, and ataxia. Perifocal edema, often severe in kidney cancer metastases, results in an increase in the brain volume in a much greater degree than the metastasis per se. Intracranial pressure increases, which clinically can manifest by headache, often diffuse, dizziness, nausea and vomiting, and congested optic discs in the study of the fundus. In some cases, sleepiness, depression, diplopia, and transient episodes of visual impairment may occur.
On CT, RCC metastases in the brain are characterized by the presence of a solid lesion with a small area of degradation in the center. CA accumulation in the solid part of the tumor is homogeneous and expressed. Lesions most often have an annular shape with clear contours and hypodense content in the center. Perifocal edema on the background of the lesion is contrasted and strongly expressed, which creates, in turn, the picture of ring enhancement. Despite the relatively slow growth of metastases, often a very pronounced perifocal edema is observed, which by size considerably exceeds the size of the metastasis. CT is required in case of the involvement of bone structures of the cranial vault, characteristic for RCC, as well as for breast and prostate cancer. Involvement of the vertebral bodies is also often identified.
The analysis of CT perfusion data showed that, on average, the blood volume (CBV) for renal cancer metastases is characterized by high values (21.01 ± 6.21 ml/100 g), indicating the presence of rich tumor vasculature. Metastatic renal cell carcinoma has relatively low CBF values (73.93 ± 27.59 ml/100 g/min) and highest MTT values (18.38 ± 1.37 s). It should be remembered that the higher the MTT values obtained, the slower the blood passes through the metastasis structure per unit of time (second). The obtained perfusion parameters allow to establish, for example, that kidney cancer metastases pass larger volumes of blood through their tissue than, for example, melanoma (CBV = 15.03 ± 9.75 ml/100 g), but have lower blood flow velocity values (CBF) and high MTT values. Thus, CT perfusion in RCC metastases to the brain allowed to establish specific perfusion parameter values—the highest CBV and MTT values among metastases and average CBF values. These characteristics allow in some extent to differentiate this type of metastases from other metastatic lesions. Despite perfusion values, similar with those for metastatic melanoma, hemorrhages in the structures of kidney cancer metastases are much rarer.
On MRI, a standard set of sequences—T1 weighted, T2 weighted, T2-FLAIR, T1 weighted + Gd in three dimensions, as well as thin-sliced SPGR (VIBE) in multiple lesions—are mandatory sequences in MR diagnosis of metastatic brain lesions. In these sequences, metastases have tissue characteristics inherent in the majority of metastases with different histogeneses. Intracerebral RCC metastases often have a rounded shape and are usually distinguished from the brain substance. They are often presented by a solid tumor, homogeneously accumulate the contrast agent, and very rarely have central necrotic changes. However, if these changes exist, their contrast enhancement prevails over that of the solid part. The visual picture of kidney cancer metastases is very similar to that of colon cancer —a “thick” roll of the solid part and a small area of decay. In 75% of cases, metastases in renal cell carcinoma are multiple.
Single-voxel MR spectroscopy did not identify any significant specificity for RCC metastases in the brain. As with other metastatic brain lesions, a marked increase in peaks of lactate and lipids is noted. The NAA (N-acetylaspartate) peak in the central structure of RCC metastases is significantly reduced.
The solid part of metastatic RCC tumors on DW images is characterized by an isointense or hypo-isointense signal. Average ADC values of the metastatic lesion are, respectively, 1.15 ± 0.9 × 10−3 mm2/s in the tumor stroma, 1.28 ± 0.2 × 10−3 mm2/s in the near peritumoral area, and 1.56 ± 0.2 × 10−3 mm2/s in the vasogenic edema area. ADC values in the tumor itself in our study were highly heterogeneous.
The use of PET with 18F-FDG for the diagnosis of primary RCC does not make much sense due to the rapid physiological accumulation of the radiopharmaceutical in the urine, with an exception of large tumors extensively extending to perirenal fat. Kidney cancer metastases, according to our data, intensely accumulate 18F-FDG in all structures of the body, including the brain. According to our data, 18F-FDG in the primary diagnosis of brain lesions can be used in a single scanning. PET with 18F-FDG showed its high sensitivity and specificity in the evaluation of the radicality of treatment; identification of a residual tumor, for example, in meningeal involvement; and differentiation of residual metastatic tumor in the brain from post-radiation changes.
15.1 Clinical Cases
See Figs. 15.1, 15.2, 15.3, 15.4, 15.5, 15.6, 15.7, 15.8, 15.9, 15.10, 15.11, 15.12, 15.13, and 15.14.
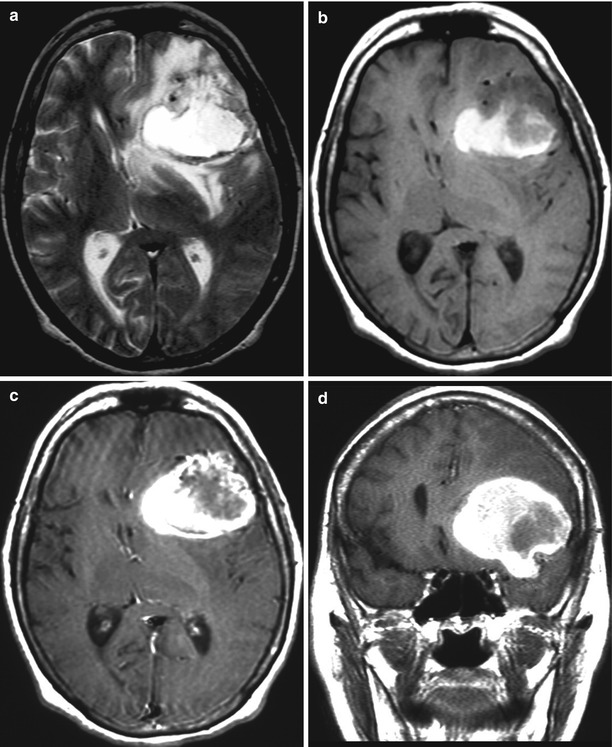
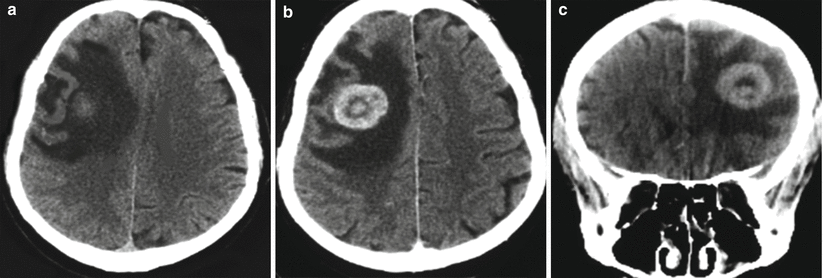
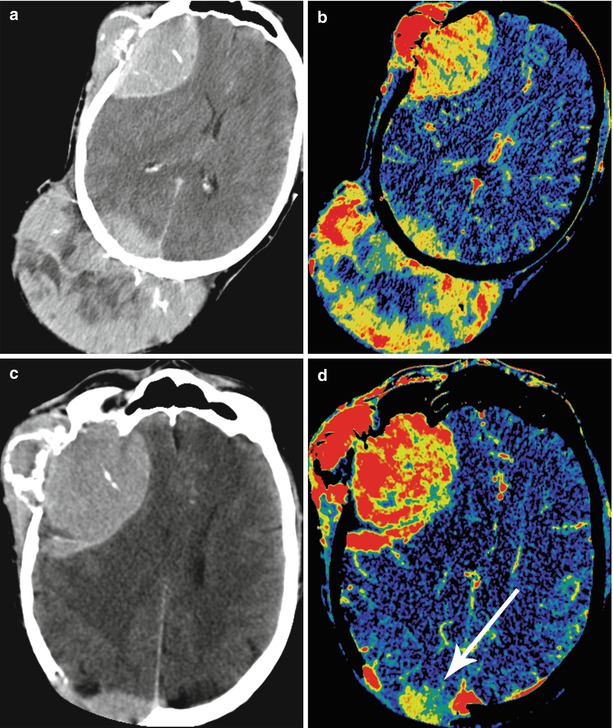
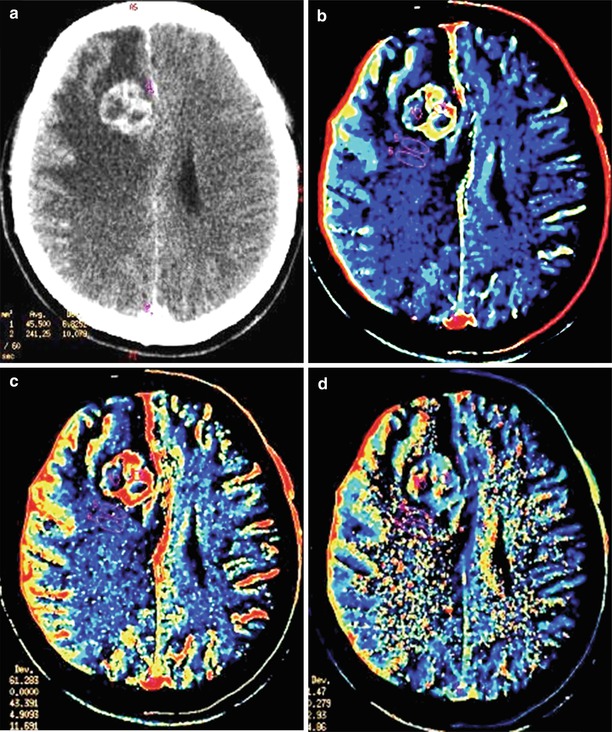

Fig. 15.1
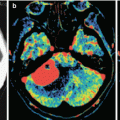
A kidney cancer metastasis in the brain. MRI: in the left frontal area, there is a large tumor site with a pronounced perifocal edema (a) and with a hemorrhagic component—a high MR signal on T1-weighted MRI (b). After intravenous CA administration on T1-weighted MRI (c, d), an area of contrast enhancement is identified around the hemorrhage, in the solid part of the tumor. The ventricular system is deformed

Fig. 15.2
A kidney cancer metastasis in the brain. In the right frontal region, there is an isodense lesion with a pronounced perifocal edema (a). Following intravenous CA administration, its pronounced accumulation in the solid part of the tumor (b, c) is observed; the typical picture of ring enhancement is observed on the background of the edema

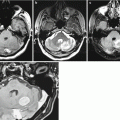
Fig. 15.3
Multiple kidney cancer metastases. A dynamic study before (a, b) and after (c, d) surgical removal. On CT images with contrast enhancement and CT perfusion maps, there are two large neoplasms with extra- and intracranial spread in the right frontal and parietal regions. The lesions are characterized by intense accumulation of the contrast agent and increased CBV values. After removal of the metastatic lesion in the right parietal region, there is a small residual tumor adjacent to the sagittal sinus (the arrow)

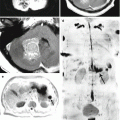
Fig. 15.4




A kidney cancer metastasis in the brain. On CT in the right frontal region, there is a solid-cystic lesion intensely accumulating the contrast agent (a) with a pronounced perifocal edema—ring enhancement. On CT perfusion, there is a significant increase in CBF (b), CBV (c), and MTT (d) values in the metastatic lesion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree