Gradea
Type
Description
I
Parenchyma
Microscopic or gross hematuria; urological studies normal (contusion)
Hematoma
Non-expanding subcapsular hematoma
II
Parenchyma
Laceration <1 cm in depth, without collecting system rupture
Hematoma
Non-expanding perirenal hematoma confined to retroperitoneum
III
Parenchyma
Laceration >1 cm in depth without collecting system rupture
IV
Parenchyma
Laceration with collecting system rupture
Vascular
Main renal artery/vein injury with contained hemorrhage
V
Parenchyma
Shattered kidney
Vascular
Avulsion of renal hilum that devascularized kidney
Table 19.2
Federle classification
Category | Type | Injury |
|---|---|---|
I | Minor injury | Renal contusion; intrarenal and subcapsular hematoma; minor laceration with limited perinephric hematoma without extension in the collecting system or medulla; small subsegmental cortical infarct |
II | Major injury | Major renal laceration through the cortex extending to the medulla or collecting system with or without urine extravasation; segmental renal infarct |
III | Catastrophic injury | Multiple renal lacerations; vascular injury involving the renal pedicle |
IV | Ureteropelvic injury | Avulsion (complete transaction); laceration (incomplete tear) |
Of course these two classification systems present several overlaps and don’t include all the possible conditions; therefore, the communication between radiologist and surgeon is of vital importance to define the grade and severity of the kidney injury.
Moreover, the AAST classification is the most and widely accepted, and it’s based on accurate assessment at autopsy, laparotomy, or radiologic study. This grading system is widely used in the urological setting. Increasing grade correlates with the need for nephrectomy and dialysis and with mortality. Grades I through III can be managed conservatively as they heal spontaneously. Grades IV and V with collecting system disruption and vascular injury usually require intervention.
This classification has some limitations, like it does not consider vascular injuries associated with low-grade injuries. There are some proposals for changes, including a sub-stratification of the intermediate-grade injury into low-risk (likely to be managed nonoperatively) and high-risk cases (likely to benefit from angiographic embolization or surgery) [23]. Another suggestion is to comprise all collecting system injuries and segmental arterial and venous injuries in grade IV injuries, while including only hilar injuries (comprising thrombotic events) in grade V injuries [24].
As we have already said, the Federle classification is based on CT findings; however, both classifications, although based on different criteria, have common points and in fact agree that the most serious lesions are those involving the excretory system and/or the vascular one.
Conservative management has become the treatment of choice for the majority of renal injuries, especially in blunt trauma [25, 26]. In particular, a nonoperative approach can be performed in hemodynamically stable patients with:
Grade I and II injuries
Most of grade III injuries
Grade IV with a devitalized fragment or with urinary extravasation
Grade V with unilateral main arterial injury, comprising unilateral complete blunt arterial thrombosis
In penetrating trauma a selective nonoperative management is generally accepted [27, 28]:
In stab wounds if the patient is stable and the site of penetration is posterior to the anterior axillary line
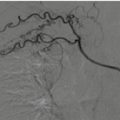
Nowadays angiography and embolization represent essential techniques in the nonsurgical treatment of traumatic kidney lesions. Superselective embolization has shown to increase significantly the chances to preserve the kidney and its function.
Embolization has a fundamental role in the conservative management of active bleeding, arteriovenous fistula, and pseudoaneurysm, and it seems to be most beneficial in the setting of high-grade renal trauma (AAST > 3).
In the management of high-grade renal trauma, embolization can be successful in up to 94.9% of grade III, 89% of grade IV, and 52% of grade V injuries and has decreased significantly nephrectomy [31–33].
Indications to operative management are limited, reserving surgery in case of shuttered kidney. The hemodynamic instability and the unresponsiveness to aggressive resuscitation due to renal hemorrhage are indications for surgical exploration, irrespective of the mode of injury [34, 35].
The exploration aims to control the hemorrhage and to save the kidney. Other indications are the mechanism of trauma; the presence of an expanding perirenal hematoma, identified at exploratory laparotomy performed for associated injuries; and the presence of multiorgan involvement which led the patient to hemodynamic instability.
Endourological techniques are indicated for the management of persistent extravasation or urinoma.
Inconclusive imaging and a preexisting abnormality or an incidentally diagnosed tumor may require surgery even after minor renal injury [36]. The overall exploration rate for blunt trauma is less than 10% [37] and may be even lower, as the conservative approach is increasingly adopted [38].
19.2 Radiological Diagnosis
The purpose of diagnostics imaging is to identify the renal lesion, to evaluate prognostic factors, and to give an indication of the patient’s management.
Currently the indications for the different diagnostic imaging modalities are controversial and depend on several things, such as the hemodynamic status of the patient, on the presence of associated lesions, and on the type and locations of the trauma.
Unstable patients are immediately examined with FAST (focused assessment with sonography for trauma), an abdominal ultrasound (US) protocol performed bedside in the emergency room for the detection of free peritoneal fluid [39].
Patients involved in a high-energy accident, in stable condition or whose vital functions have been stabilized, are rapidly examined with a whole-body computed tomography (CT) [40].
The management of patients with mild-/low-energy trauma is controversial: the clinical presentation and the mechanism of injury are fundamental for the decision to immediately perform CT or assess the patient with sonography, conventional radiography, and clinical observation [41–44].
In 2014 the American Urological Association (AUA) released new guidelines, amended in 2017 [45], for management of patients with a suspect of renal trauma.
CT with administration of intravenous contrast material is recommended in adults with blunt trauma and one of the following cases [2, 10, 46, 47]:
Gross hematuria: represents the main initial indicator of a significant renal lesion, although it is not correlated with the degree of injury
Microscopic hematuria in the presence of shock (systolic blood pressure < 90 mmHg)
A mechanism of injury (e.g., rapid deceleration or high-speed collisions)
A physical examination concerning for renal injury: contusion or flank ecchymosis, fracture of the last ribs or thoracolumbar spine, and open wound of the abdomen, of the flank, or of the lower part of the thorax, in case of expanding mass of the flank that may be a hematoma or urinoma, regardless of the presence or absence of hematuria
In case of retroperitoneal fluid, nausea, vomit, or paralyzed ileum
A diagnostic evaluation is mandatory also in all cases of penetrating traumas, because in those situations there is a poor correlation between the presence of hematuria and the severity of the injuries [48].
Gross or microscopic hematuria is usually present in 95% of renal trauma, but its absence doesn’t preclude the presence of kidney injure, for example, it can be absent in 24% of patients with renal artery thrombosis, and in approximately 30% of urinary tract junction lesions, these lesions, moreover, are major injuries.
Although no exact indications have been given in the AUA 2014 guidelines for patients with penetrating renal trauma, the most accepted one is to perform a CT in the presence of hematuria or in a clinical suspect of a urinary tract lesion.
The Urogenital Trauma guidelines of European Association of Urology (EAU) released in 2013 and updated until 2017 are similar to the AUA ones [2, 46].
Some authors affirm that patients with microscopic hematuria and systolic blood pressure >90 mmHg have a very low risk of major renal injury (incidence of 0.2%) [48], so they could not require a diagnostic imaging evaluation.
Renal injuries, especially high-grade ones, seem to be more frequent in children, and these can also occur for minor trauma [49]; this is because of the anatomy of pediatric patient. In fact, the kidney in children is larger compared to the rest of the body, and it can maintain fetal lobulation that could easier lead to parenchymal disruption; a child’s kidney is also less protected because it has less perirenal fat, and the abdominal wall thickness is less than that of the adult [37, 50].
In pediatric patients the diagnostic imaging choice is controverted. Due to the capability of children to maintain their blood pressure, instead of adults, some centers recommend a CT scan in case of suspected renal involvement in pediatric blunt trauma with any degree of hematuria following significant abdominal trauma.
In pediatric blunt renal trauma, CT is indicated when the RBC value in the urine is >50 per HPF and in penetrating trauma when the RBC value in the urine is >5 per HPF [10].
19.2.1 Ultrasonography (US)
Ultrasound, just for its well-known advantages, which consist of low cost, lack of ionizing radiations, and its portability with the possibility to be rapidly performed at patient’s bedside, among others, is the most largely available imaging modality in emergency department.
Apart from its use in the emergency room with FAST (focused assessment with sonography for trauma) to highlight the presence of hemoperitoneum, ultrasound is often the first imaging of choice in evaluating a patient with localized low-energy trauma. In fact a standard ultrasound technique is able not only to detect free fluid but also to demonstrate a parenchymal lesion. In the specific case of kidney, the deep retroperitoneal position and the body type of patient can influence the detection of the lesion; moreover, the operator’s experience and patient’s collaboration are other factors that may affect the outcome of the exam. For these reasons US demonstrates high sensitivity for the detection of free intra-abdominal fluid; in the same study, it is reported more than CT exam for small amount, but fairly low sensitivity (even below 50%) for the detection of abdominal solid organ traumatic lesions [51].
Some studies report that the US, practiced in an emergency environment, has very low sensitivity in the detection of parenchymal renal injury (less than 22% in minor lesion) and perirenal collections [51–55]. The American College of Radiologists (ACR) Renal Trauma guidelines consider US usually not appropriate in renal trauma [46]. It also cannot be a reliable diagnostic tool for major vascular injuries and renal function.
Contrast-enhanced ultrasound (CEUS) in traumatic patients has been shown to be more sensitive than US for the detection of solid organ injuries, improving the identification and grading of traumatic abdominal lesions with levels of sensitivity and specificity similar to CT (up to 95%) [56]. With CEUS is also easier demonstrated even small amount of perirenal fluid and sometimes is possible to detect active bleeding. The principal limit of CEUS is the impossibility to evaluate the excretory phase, because microbubbles are not excreted into the collecting system; therefore, CEUS cannot demonstrate injuries to the urinary system: renal pelvis or ureter [57–60].
For these reasons both basic ultrasound and CEUS may not be the only investigations to evaluate a patient with trauma. Their role, especially that of CEUS, is to identify patients with a positive traumatic injury response and to send them a CT diagnostic completion test, differentiating them from negative traumatic injury patients who, if in agreement with the clinic, cannot continue the radiological diagnostic iter.
In this way it decreases the unnecessary radiation exposure, using the US-CEUS as a screening tool to select patients who require a CT or not.
This is very important especially in selected series of patients, such as pediatric patients, young women in reproductive age, and low-energy trauma patients, or in the follow-up of stable patients with kidney injury [39, 41, 61].
An US exam is performed using a convex-array multifrequency 3.5–8 MHz probe. During the US exam, a parenchymal renal injury can be seen as a slightly hyperechoic area with no defined margins that may be difficult to detect in renal parenchyma. Often even in case of minor trauma a surrounding hematoma is visible, as well as a recent hyperechogeneous hematoma, which sometimes can be confused with renal parenchyma (Fig. 19.1).


Fig. 19.1
Ultrasound shows injured left kidney, with a hyperechoic parenchymal area at the medium of the kidney, corresponding to the lesion (arrow); there is a hyperechoic hematoma surrounding the kidney (arrowhead)
Over time, hematoma loses its echogenicity, becoming hypo-anecdotal and decreasing its volume (Fig. 19.2).


Fig. 19.2
Ultrasound shows a non-recent subcapsular hematoma, which appears as an hypoechoic mass compressing renal parenchyma
Usually CEUS exam, in case of low-energy localized renal trauma or during a follow-up of known injuries, is performed using a convex-array multifrequency 3.5–8 MHz probe after a previous basal ultrasound. CEUS uses second-generation ultrasound contrast agents and needs dedicated software operating at low mechanical index.
Like any contrastographic examination, the informed consent of the patient is required.
After contrast agent administration with quick bolus, the renal cortex enhances immediately in arterial phase (10–30 s), very brightly and evenly, and the pyramids enhance diffusely from the periphery to the center over about 30 s. The homogeneous phase of the kidneys generally lasts 2–2.5 min: this homogeneous phase (venous phase or nephrographic phase) is still the most effective for detection of traumatic injuries. At CEUS exam the injured area is detected as anechoic surrounded by normal strongly hyperechogenic renal parenchyma (Fig. 19.3). Perirenal or subcapsular hematoma is easily seen as perirenal hypo-anechoic zone, in case of subcapsular hematoma, with the typical imprint on the kidney profile (Fig. 19.4). In case of active bleeding, it is possible to detect in the injured area the hyperechoic spots. As we already said, it is impossible evaluate the excretory system [53, 60].



Fig. 19.3
CEUS, axial view of the kidney, shows the renal injury as linear anechoic area. Note the small perirenal hematoma as a subtle fluid collection surrounding the kidney (arrowhead)

Fig. 19.4
CEUS shows a deep laceration of renal parenchyma (black arrow). Perinephric hematoma is seen as a hypoechoic fluid collection surrounding the kidney (arrowheads)
When a renal lesion is detected at US or CEUS complete, the examination of the patient performing a CT with intravenous contrast medium is recommended.
Eco-color-Doppler imaging can be useful in monitoring vascular posttraumatic complications such as pseudoaneurysms, arteriovenous fistulas, and arterial or venous renal thrombosis.
19.2.2 Multidetector Computed Tomography (MDCT)
With the technological development in the past two decades, contrast-enhanced MDCT has gained a central role in the evaluation of stable polytrauma patients, becoming the first choice examination. Integration of whole-body CT into the initial management of polytrauma patients significantly increases the probability of survival and a better prognosis [62].
CT can quickly and accurately identify and grade renal injury [63], can establish the condition of the contralateral kidney, and can demonstrate injuries to other organs.
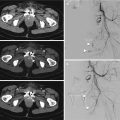
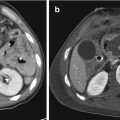
In the setting of renal trauma, multiphase CT allows the most comprehensive assessment of the injured kidney. The standard protocol consists in an abdominal pre-contrast acquisition from the diaphragm to the pubic symphysis, followed by a post-intravenous contrast exam in arterial phase (delay around 40 s) and venous nephrographic phase (delay around 80 s). A pyelographic phase (at 5–10 min or more) is practiced only in the suspected urinary tract injury, e.g., in the presence of collections to differentiate an active bleeding from a urinoma (Fig. 19.5) [64, 65].


Fig. 19.5
CT exam in a patient with a urine leakage from pyelo-ureteral tract, with huge urinoma. (a) Arterial phase and (b) axial and (c) coronal MPR in excretory phase. Note the importance of the late excretory phase that allows to differentiate a perirenal collection, e.g., hematoma from urinoma
A volume of nonionic contrast medium of 100–150 mL is injected at a rate of 2–4 mL/s through an 18–20-gauge needle.
Concerns regarding contrast media worsening outcomes via renal parenchymal toxicity are likely unwarranted, with low rates of contrast-induced nephropathy seen in trauma patients [66].
However, in practice, trauma patients usually undergo standardized whole-body imaging protocols; it may happen that, caused by critical patient’s condition, it is not possible to perform an excretory phase; and in this case if there is suspicion that renal injuries have not been fully evaluated, repeating renal imaging when it is possible should be considered.
19.2.3 Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is not commonly used imaging modality in trauma patients, due to the time needed for examination, the difficulty to manage a traumatized patient in MR room, the limited access to the patient during the acquisition of imaging, the need for MRI-safe equipment, and the logistical challenges of moving a trauma patient to the MRI suite. Anyway the diagnostic accuracy of MRI in renal trauma is similar to that of CT with the benefit due to the lack of radiations exposure [67, 68]. Due to the lack of radiations, MRI can be useful, especially in the assessment of pediatric patients and young women, in cases in which the use of iodine contrast material is contraindicated or in follow-up of renal and urinary tract lesions, since with the administration of gadolinium the extravasations of urine can be visualized [69–71].
19.2.4 Urography, Angiography, and Scintigraphy
Intravenous urography is nowadays an obsolete imaging modality for the evaluation of renal trauma. It can be used only in rare situations, if the MDCT is not available or in the operating room, in hemodynamically unstable patients taken to the operating room without imaging, to confirm the contralateral renal function if nephrectomy is considered. The technique consists of an injection of intravenous contrast (2 mL/kg) followed by a single plain film taken after 10–15 min [46, 72]. In doubt or positive cases, MDCT is anyway necessary once the patient is stable.
Angiography has only a therapeutic role in renal vascular lesions in stable patients. This topic will be treated in Chap. 22.
Kidney scintigraphy with Tc-99m glucoheptonate, Tc-99m mercaptoacetyltriglycine, or Tc-99m-diethylenetriamine penta-acetic acid can be useful in the follow-up of renal injuries, to counsel the patient on the expected renal function [65].
It can be used also in studying the renal function in patients with contraindications to contrast media or in very selected cases.
19.3 Renal Trauma Classification
Renal trauma management depends widely not only on the detection of renal parenchyma lesion but also on the presence of bleeding parenchymal lesions or direct damage to the vascular peduncle or compromise of the urinary excretory system. The presence of these kinds of injury, detected at CT exam, completely changes management from nonoperative to operative. The CT exam with intravenous contrast medium can show accurately not only the presence of a renal injury and its grade but also can allow the identification of a preexisting renal condition mimicking trauma and can explore the contralateral kidney and the presence of concomitant lesions of other organs.
Therefore the radiologist plays an essential role in the management of traumatic patient and regarding renal trauma distinguishing kidney lesions that need interventional/surgical treatment from the ones needing only a conservative approach.
Since this manuscript is mainly intended for an imaging-based audience, we take into consideration the Federle classification dividing traumatic renal injury in two principal categories of injury: minor and major—anyway the two principal classifications will be considered for the description of the different kidney lesion.
19.3.1 Category I: Minor Traumatic Lesions
This category comprises minor renal contusions and lacerations which don’t extend to the collecting system or medulla, subcapsular hematomas with less than 1 cm or more than 1 cm of thickness but without urinary excretal delay, and perinephric hematomas without active bleeding comprised in the perinephric adipose space and small subsegmental cortical infarcts.
Category I corresponds in the AAST renal injury scale to grades I and II. It includes most of kidney injuries (75–85%), which generally are treated conservatively.
19.3.1.1 Imaging Findings
Renal Contusion
Contusion represents a self-limiting blood extravasation (hematoma) in the renal parenchyma (grade I AAST; type I Federle). These minor injuries will spontaneous resolve and follow-up imaging is not required.
At an early US examination (within 1 h from trauma), it can appear as an oval or round hyperechoic area, with margins that after being undefined at the beginning become more and more distinct; rarely it is large enough to lead to a mass effect, alter the cortical profile, or determine a dilatation of nearby chalices (Fig. 19.6). The echogenicity of renal contusions reduces in a few days until becoming isoechoic within 2 weeks, generally without leaving sequelae. Anyway contusion has to be suspected when a trauma patient presents hematuria without significant alterations or abnormalities of the urinary tract at US. On CEUS kidney contusion lesion can appear as a hypoechoic area without clear delimitation [50, 53, 59, 71].


Fig. 19.6
Minor lesion: US shows the lower pole renal contusion as a non-well-defined inhomogeneous parenchymal (arrow); note the surrounding perirenal hematoma as a hyperdense collection (arrowheads), not compressing the renal profile
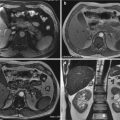
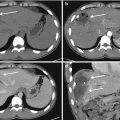
Compared to US, MDCT is more sensible in detecting renal contusion. On unenhanced images, it may be slightly hyperdense (due to the accumulation of acute blood products); in enhanced phases contusion appears as an ill-defined hypoattenuating lesion. Sometimes renal contusions can be seen as focal areas of striated nephrogram and as hyperdense areas in the excretory phase, due to small iodinated urine extravasations in the intraparenchymal collecting system. These lesions have to be distinct from segmental infarction that is usually linear or wedge-shaped sharply defined non-enhancing areas [21, 64, 65, 73, 74] (Fig. 19.7).


Fig. 19.7
Renal contusion seen on MDCT. Unenhanced CT scan (a) shows an iso-hyperdense area, which is better delineated on post-contrast images (b) and coronal reconstruction (c) as a hypodense area
Subcapsular Hematoma
Subcapsular hematoma is a collection of clotted blood situated under the renal capsule. It is quite rare due to the tight adherence of renal capsule and cortex (grade I AAST; type I Federle). On US it is seen at the beginning as hyperechoic lenticular lesion, which is distributed along the external kidney surface, confined between the cortex and the capsule, determining a compressive effect on the renal parenchyma. Over time, it becomes a hypo-/anechoic lesion and reduces its thickness. On CEUS a subcapsular hematoma appears as a nonhomogeneous fluid collection without enhancement surrounding the kidney [41, 43, 53, 59].
On CT, acute hematomas are seen as a round or elliptical fluid hyperdense collection (>35–55 UH) [21, 64] on unenhanced scan, with an oval or crescent shape which imprints the underlying renal parenchyma; if the fluid collection is of greater size, it can have a biconvex shape and causes a delay in the nephrographic phase (Fig. 19.8). The Federle classification, unlike the AAST, evaluates the presence of any delay or reduction of parenchymal vascularity due to hematoma compression. In case of a chronic and prolonged compression and distortion of the renal parenchyma and vessels, a reduction of the blood flow to the kidney occurs resulting in activation of the renin-angiotensin system with development of hypertension, also known as “Page kidney phenomenon.” Surgical treatment of the renal compression is indicated in these cases [21, 64].


Fig. 19.8
Subcapsular hematoma seen on MDCT unenhanced scan (a) as a hyperdense area, without expanding signs on post-contrast images (b and c)
Superficial and Deep Laceration
Superficial renal laceration (< 1 cm depth) is a tear of the renal parenchyma that involves only the cortical zone, often associated with contusion. Instead a deep laceration (>1 cm depth) passes through the cortical zone extending to the medullary one. A laceration appears as irregular or linear parenchymal defects that may contain clot (grade I–III AAST; type I Federle) [74].
Superficial or deep renal lacerations appear as defects in the renal parenchyma without involvement of the collecting system and typically resolve spontaneously, without the need for follow-up imaging, especially in case of superficial laceration [21]. If it extends only to the renal cortex, it is classified as AAST grade II, and if it goes until the medullar part, without comprising the collecting system, it is classified as AAST grade III and may need follow-up imaging.
On US it is difficult to observe, and it can be suspected if a subcapsular or perirenal hematoma is seen; it can be evident as a hyperechoic line or an undefined area of the kidney profile. On CEUS laceration is seen as a linear or branched hypoechoic streak, perpendicular to the surface of the kidney [59] (Fig. 19.9). The rapid enhancement can generate questions of interpretation that can possibly be solved only with a second injection of contrast agent [75]. An injection of too high a dose of contrast media will have a negative effect due to the intense enhancement, potentially masking the presence of lacerations [61].


Fig. 19.9
(a) US shows a large perirenal hematoma (arrowhead); it isn’t appreciable the renal parenchymal injury. (b) CEUS demonstrates a deep parenchymal laceration at the lower pole of the kidney (arrow)
On MDCT it is seen as a hypodense linear or irregular streak on unenhanced scan; after contrast material administration, it is visualized as a less or unenhanced area; therefore, sometimes the differential diagnosis with a segmental or subsegmental infarct can be difficult (Fig. 19.10).


Fig. 19.10
Superficial laceration seen as a hypodense streak on post-contrast images (a), better visualized on 3D (b) and sagittal (c) reconstructions
Perirenal Hematoma
Perirenal hematoma is a hemorrhagic extravasation in the perirenal adipose tissue, within Gerota’s fascia, which normally represents the result of a laceration of the renal capsule. Usually it is not creating a distortion of the kidney’s profile (from grade II AAST; type I Federle). This kind of finding is treated conservatively. Sometimes the hematoma can be very large and dislocate the kidney.
Ultrasound is not sensible in distinguishing between acute hematoma and the perirenal fat tissue density, since both are hyperechoic. On CEUS the adipose tissue shows a low enhancement and can therefore be differentiated from the non-enhancing fluid collection.
On MDCT in basal phase, an acute perirenal hematoma is seen as a hyperdense collection more irregular in shape than subcapsular hematoma. The perirenal fascia can be thickened if it is infiltrated by the hematic collection [18, 65]. After contrast intravenous medium, the hematoma will be hypodense; in case of active bleeding, hyperdense foci or pooling of contrast medium will be seen in the hematoma (Fig. 19.11).