Fig. 17.1
A 48-year-old man with melanoma. An axial CT image of the abdomen reveals a hypervascular mass in the right kidney. A biopsy revealed renal cell carcinoma
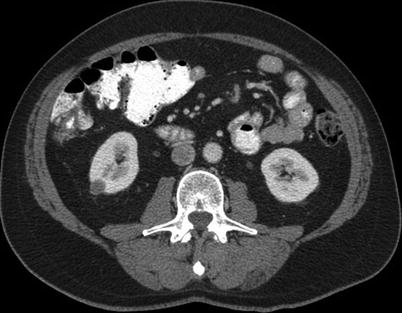
Fig. 17.2
A 64-year-old man with a history of prostate cancer. A CT image of the abdomen reveals a low-density mass in the right kidney. A biopsy revealed renal cell carcinoma
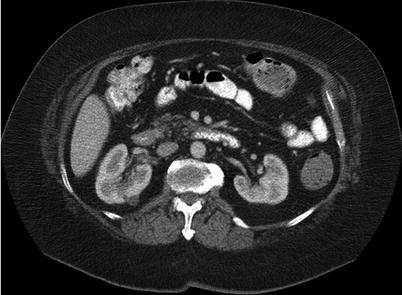
Fig. 17.3
A 64-year-old woman with primary peritoneal carcinoma. A CT image of the abdomen reveals a mass in the right kidney. A biopsy revealed metastatic disease
In some reported autopsy series of patients with lymphoma, the kidneys were found to be involved in 34–60 % of cases [15]. However, renal lymphoma is detected on routine abdominal imaging in only 2.7–15 % of patients who undergo computed tomography (CT) scans during lymphoma staging investigation [16, 17]. The discrepancy between actual cases and imaging-detected cases of renal lymphoma during staging may be due to variations in the radiological appearance of renal lymphoma; on imaging, the appearance of renal lymphoma can vary from a well-defined mass resembling primary renal tumors to diffuse involvement and enlargement of the kidney [18]. Image-guided biopsy can help differentiate renal lymphoma from epithelial tumors of the kidney (Fig. 17.4). Patients with renal lymphoma are treated with chemotherapy; surgery or ablation does not have a role in treating these patients.
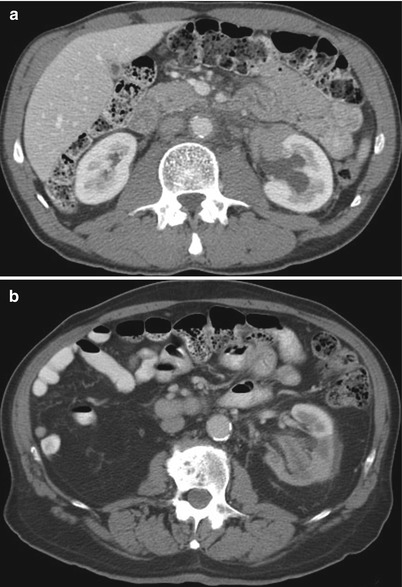

Fig. 17.4
(a) A 58-year-old man with a history of lymphoma was found to have a left renal mass. A biopsy revealed lymphoma. (b) A CT of the abdomen in an 86-year-old man with a history of lymphoma reveals an infiltrative left renal mass. A biopsy revealed urothelial carcinoma
Patients with Unresectable RCC
RCC is a heterogeneous malignancy and has several subtypes that exhibit distinct clinical and histological features [19, 20]. In patients who have an unresectable renal mass, who are poor surgical candidates, or who have metastatic RCC, renal mass biopsy can establish the diagnosis so that optimal treatment and/or treatment sequence can be determined [11] (Figs. 17.5 and 17.6). This is particularly important in the current era of molecular targeted therapy, where several agents with distinct mechanisms of action are now available to treat patients with advanced RCC [21].
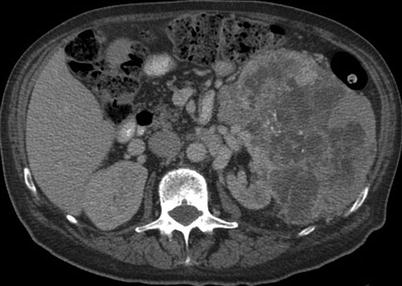
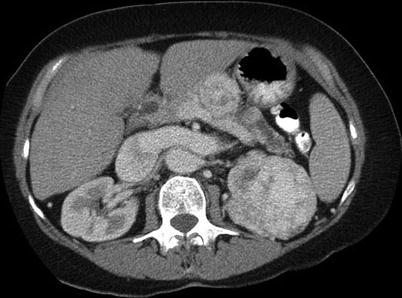

Fig. 17.5
A 67-year-old man with a large renal mass. A biopsy of the left renal tumor revealed renal cell carcinoma. He underwent cytoreductive nephrectomy

Fig. 17.6
A 63-year-old woman with a left renal tumor underwent biopsy. A pathologic evaluation revealed renal cell carcinoma. She was treated with radical nephrectomy
Patients with a Renal Mass and Febrile Urinary Tract Infection
Renal infections such as focal bacterial infections and xanthogranulomatous pyelonephritis can present as mass-like lesions on imaging studies [22–24], although ill-defined margins and perinephric stranding may suggest an infectious condition rather than a neoplastic process [25, 26]. In patients with clinical signs and symptoms of urinary tract infection and a focal mass-like lesion shown on imaging, appropriate antibiotic therapy and follow-up imaging may obviate biopsy; however, if the mass persists, image-guided biopsy is indicated to confirm an infectious process and to rule out renal cancer.
Patients with an Indeterminate Cystic Renal Mass
Complex cystic masses present a diagnostic and management dilemma. Approximately 10–15 % of RCCs have cystic components on cross-sectional imaging [27]. The Bosniak renal cyst classification system categorizes cystic lesions of the kidney into one of five groups: categories I, II, IIF (F: follow-up), III, and IV (Table 17.1) [28]. According to this classification system, cystic lesions in categories I and II are benign and do not require further evaluation or intervention (Figs. 17.7 and 17.8). Although lesions in category III are “indeterminate,” they are worrisome enough to warrant a recommendation for surgical excision (Fig. 17.9). Category IV lesions are clearly malignant on the basis of their imaging characteristics and require surgical removal (Fig. 17.10). Category IIF was added to the original Bosniak classification system because some category II cysts were more complicated than expected yet were not complicated enough to warrant placement in category III. Lesions classified as IIF require follow-up imaging studies to confirm benign status.
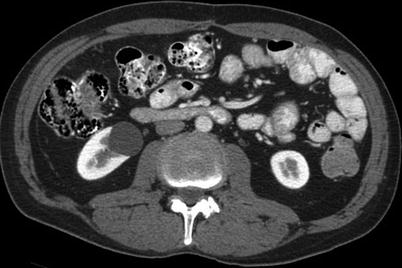
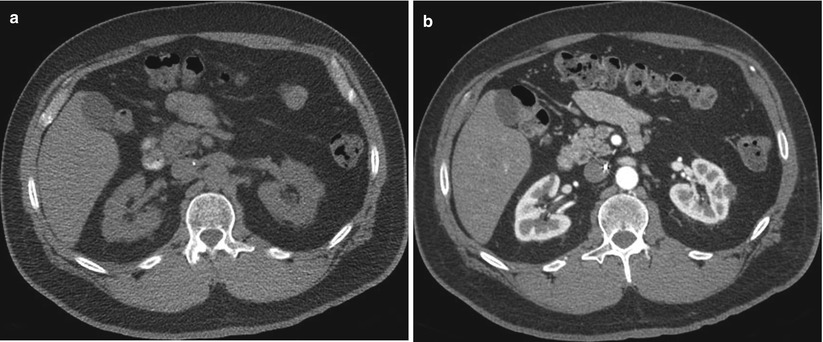
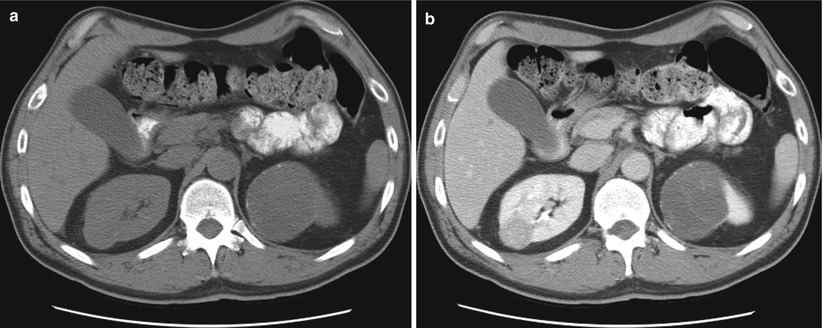
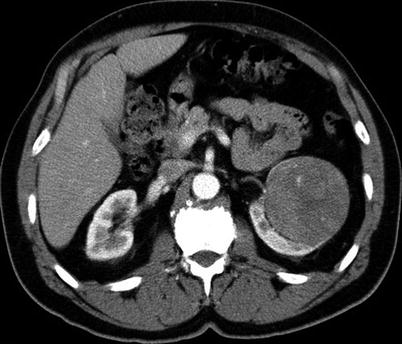
Table 17.1
Bosniak renal cyst classification system
Category | Description |
|---|---|
I | A benign simple cyst with a hairline thin wall that does not contain septa, calcifications, or solid components. It measures water density and does not enhance. |
II | A benign cyst that may contain a few hairline thin septa in which “perceived” enhancementa may be present. Fine calcification or a short segment of slightly thickened calcification may be present in the wall or septa. Uniformly high-attenuation lesions <3 cm (so-called high-density cysts) that are well marginated and do not enhance are included in this group. Cysts in this category do not require further evaluation. |
IIF | Cysts that may contain multiple hairline thin septa or minimal smooth thickening of their wall or septa. Perceived enhancement of their septa or wall may be present. Their wall or septa may contain calcification that may be thick and nodular, but no measurable contrast enhancement is present. These lesions are generally well marginated. Totally, intrarenal nonenhancing high-attenuation renal lesions >3 cm are also included in this category. These lesions require follow-up studies to prove benignity. |
III | “Indeterminate” cystic masses that have thickened irregular or smooth walls or septa in which measurable enhancement is present. These are surgical lesions, although some will prove to be benign (e.g., hemorrhagic cysts, chronic infected cysts, and multiloculated cystic nephroma) and some will be malignant, such as cystic renal cell carcinoma and multiloculated cystic renal cell carcinoma. |
IV | These are clearly malignant cystic masses that can have all the criteria of category III but also contain enhancing soft tissue components adjacent to, but independent of, the wall or septum. These lesions include cystic carcinomas and require surgical removal. |

Fig. 17.7
A CT image of the abdomen in a 60-year-old man with a history of gastrointestinal stromal tumor reveals a simple renal cyst in the right kidney

Fig. 17.8
An axial CT image of the abdomen in a 56-year-old man with pancreatic neuroendocrine carcinoma reveals a small hemorrhagic renal cyst in the lateral aspect of the left kidney (a). There was no enhancement after administration of intravenous contrast material (b)

Fig. 17.9
Axial CT images before (a) and after (b) intravenous contrast administration reveal a solid enhancing mass in the right kidney and a Bosniak III cyst involving the upper pole of the left kidney. The left upper pole cystic mass was resected. A pathologic evaluation revealed renal cell carcinoma (60 % clear cells, 40 % eosinophilic cells, Fuhrman nuclear grade 3). A fine-needle aspiration biopsy of the right renal tumor was performed at the time of ablation. A pathologic evaluation revealed renal cell carcinoma. The subtype and nuclear grade could not be determined from the fine-needle aspiration biopsy sample

Fig. 17.10
A 72-year-old man with prostate cancer was found to have a complex solid and cystic mass in his left kidney, Bosniak class IV. A biopsy revealed renal cell carcinoma
Historically, percutaneous biopsy has not been used to evaluate complex cystic renal masses. It is generally believed that a benign diagnosis can only be established after examination of the entire lesion, and a biopsy specimen that does not contain malignant cells does not rule out the possibility of malignancy. Therefore, category III cystic lesions should be surgically excised. On the other hand, several reports have been published that support using percutaneous biopsy to evaluate an indeterminate cystic renal mass [27, 29, 30]. The authors of these reports argued that percutaneous biopsy can help identify benign causes of indeterminate cystic renal masses, thus obviating surgery in a subset of patients. The major problem with these studies is that it is very difficult to prove negative results [7]. Sampling error remains a potential limitation of biopsy in cases where the cancer may be focal and interspersed with benign cystic spaces. Therefore, biopsy of complex cystic renal masses is probably helpful only in selected cases in which the risk of surgical resection is too high or the patient is considered a candidate for thermal ablative therapies (Fig. 17.11).
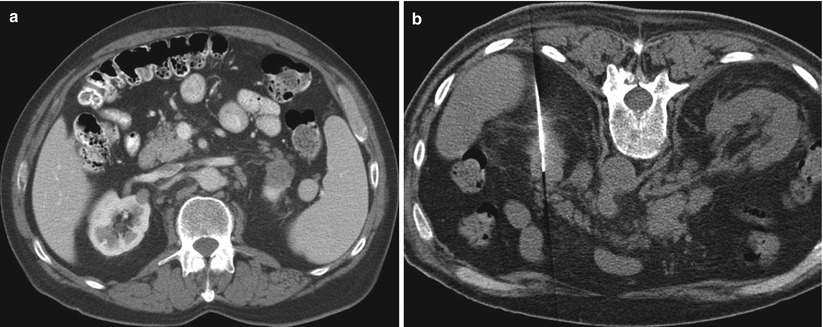

Fig. 17.11
An axial CT image of the abdomen (a) in a 70-year-old man with a history of lymphoma reveals a small cyst in the right kidney and a complex cystic mass in the upper pole of the left kidney. A CT-guided biopsy (b) of the left upper pole renal mass revealed renal cell carcinoma
Patients with a Solid Renal Mass
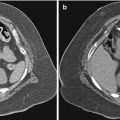
Traditionally, most solid enhancing renal masses have been presumed to be primary renal cancer. When a patient presents with a locally invasive renal mass or metastatic disease that is thought to be inoperable, percutaneous needle biopsy is performed to confirm the diagnosis prior to initiation of systemic therapy. Historically, if disease was localized to the kidney and was deemed resectable, preoperative percutaneous needle biopsy was not performed because of concerns about complications and the accuracy of biopsy results [31]. With the widespread use of cross-sectional abdominal imaging (e.g., CT, ultrasonography, and magnetic resonance imaging [MRI]), most renal tumors are now detected incidentally when they are still small and are confined to the kidney [2]. It is now recognized that small renal masses represent a heterogeneous group of pathologic entities, ranging from benign to malignant. When resected without a preoperative diagnosis, 23 % of tumors smaller than 4 cm were found to be benign (Fig. 17.12) [3]. For smaller tumors, the incidence of benign pathology is even higher. When tumors smaller than 1 cm were resected, 46 % were found to be benign. Aside from traditional nephrectomy, several new treatment options for small renal tumors are now available. Active surveillance, image-guided ablation, and partial nephrectomy are some of the less invasive treatment options that have gained varying degrees of acceptance in the urologic community. As a result, there has been renewed interest in percutaneous image-guided needle biopsy of small renal masses to help guide treatment of these patients (Fig. 17.13). Pretreatment percutaneous biopsy has been shown to decrease the number of unnecessary surgeries for benign disease in 16–44 % of cases [32, 33].
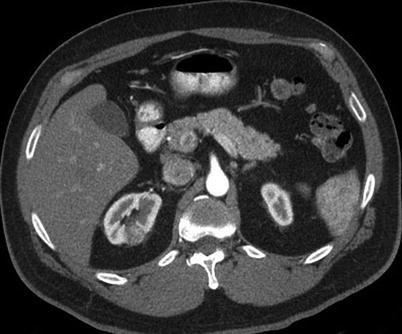
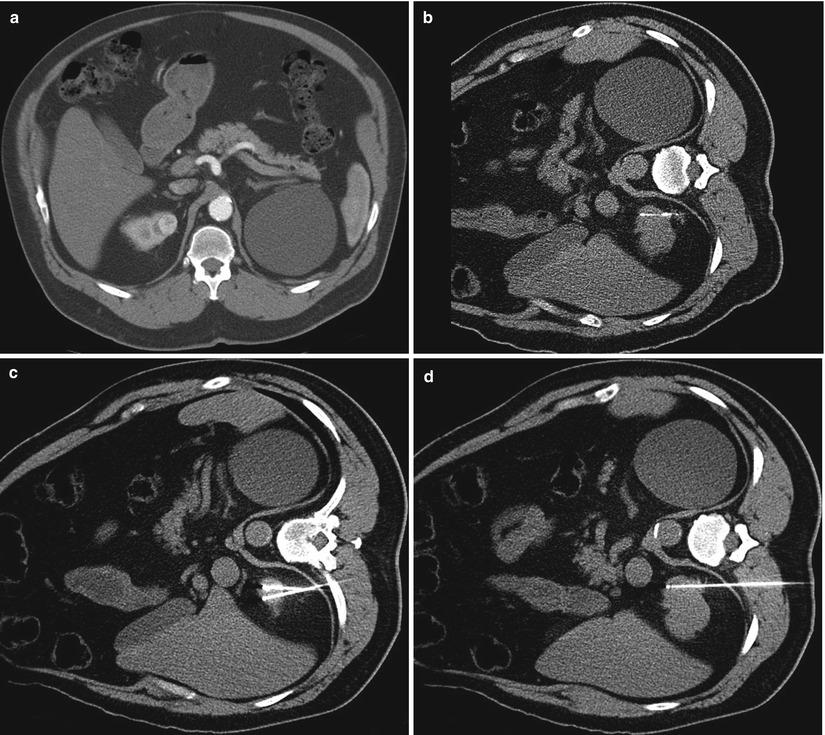

Fig. 17.12
A 56-year-old man with pancreatic neuroendocrine carcinoma was referred for evaluation of a solid enhancing mass involving the right kidney. An axial CT image reveals a 2-cm right renal mass; a biopsy revealed oncocytoma

Fig. 17.13
A 62-year-old man with a history of esophageal cancer was found to have a solid enhancing mass in the upper pole of his right kidney (a). A CT-guided biopsy (b) was performed in the lateral decubitus position. A histological evaluation revealed renal cell carcinoma. He subsequently underwent percutaneous radiofrequency ablation. Axial CT images during the ablation (c, d) reveal three radiofrequency electrodes in the tumor
Patients with Suspected RCC Managed with Ablation Therapy
In the recent years, ablation therapies have evolved as an alternative treatment option for patients with small RCCs who may not be suitable candidates for surgery [34–37]. Unlike surgical excision in which the harvested tumor undergoes pathologic evaluation, the ablated tumor remains in situ after the procedure, precluding pathologic assessment. Because a substantial number of small renal masses are benign, some researchers have suggested that a pre-ablation biopsy should be performed to establish a diagnosis [38]. After the ablation, surveillance imaging studies are conducted to confirm complete ablation of the tumor and lack of recurrence [39]. The lack of enhancement after administration of contrast on CT or MRI is confirmation of technical and clinical success (Fig. 17.14). Over time, the ablation zone will undergo involution, resulting in “shrinkage” of the ablated tumor (Fig. 17.15). Any abnormal enhancement or enlargement of the ablation zone may indicate residual or recurrent tumor and should be further investigated via percutaneous biopsy (Fig. 17.16) [40]. Some researchers have advocated a routine biopsy of the ablated tumor to confirm the absence of any viable cancer cells in the ablation zone [37, 41]. Neither one of these strategies is free of pitfalls. Particularly in cases of radiofrequency ablation wherein the cellular architecture is preserved for some time after ablation, hematoxylin and eosin staining can be misleading with false-positive results [42]. Viability staining (e.g., nicotinamide adenine dinucleotide hydrogenase) may be necessary to confirm complete ablation of the tumor. At the time of this writing, a biopsy of the ablation zone is indicated if any signs of enhancement or enlargement or any unusual or unexpected findings are present on surveillance imaging studies [40].
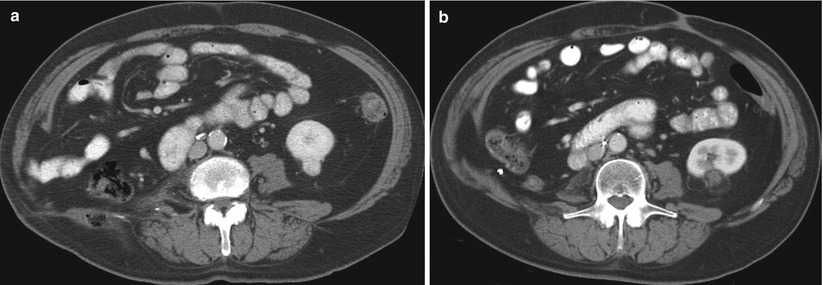
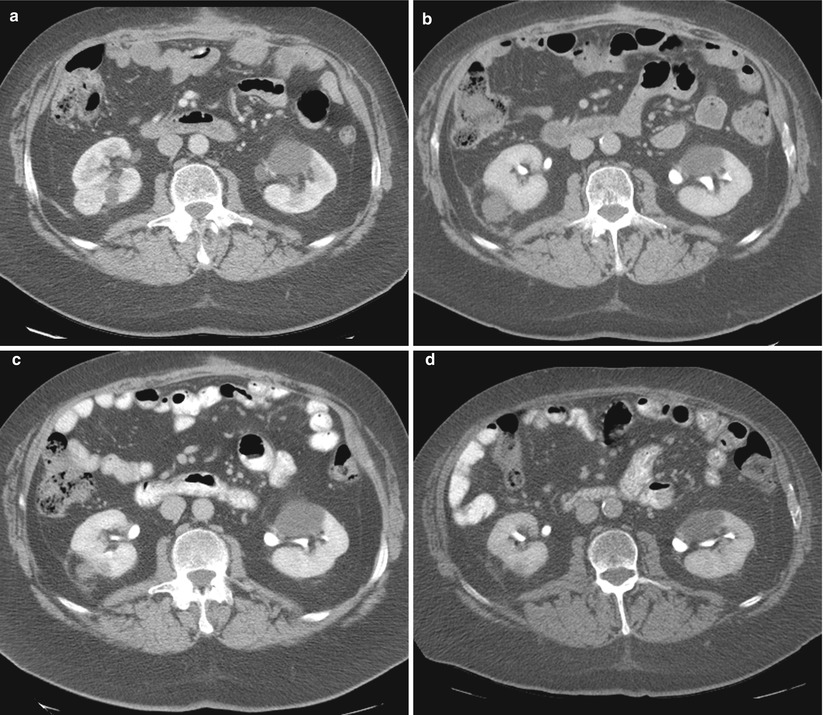
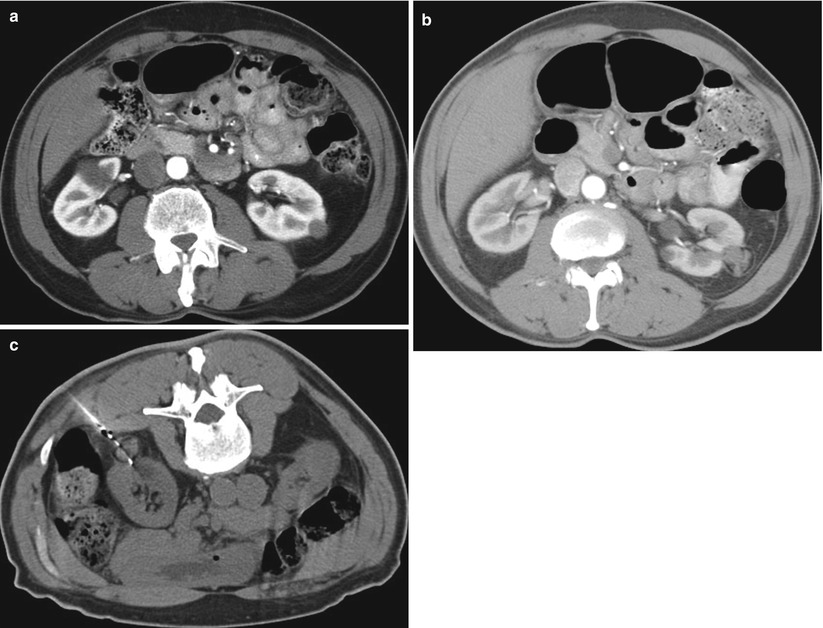

Fig. 17.14
A 77-year-old man with a history of renal cell carcinoma and right nephrectomy was referred for treatment of a new enhancing renal mass involving the lower pole of his left kidney (a). An axial CT image of the left kidney 1 year after ablation reveals no enhancement in the tumor (b)

Fig. 17.15
A 68-year-old man underwent cryoablation of a solid enhancing tumor in the posterolateral aspect of the right kidney (a). Follow-up CT images at 1 month (b), 6 months (c), and 29 months (d) reveal progressive involution of the ablated tumor and the ablation zone. At 29 months, the tumor has nearly completely disappeared. Tumors treated with radiofrequency ablation demonstrate lesser degrees of involution

Fig. 17.16
A 66-year-old man with a small left renal cell carcinoma was referred for percutaneous ablation. An axial CT image of the abdomen (a) reveals a solid, low-density, left renal tumor. Follow-up CT images at 1 and 6 months (not shown) demonstrated continued involution of the ablation zone. A repeat CT study at 1 year (b) reveals enlarging soft tissue density adjacent to the tumor in the ablation zone. The findings were suspicious for a recurrent tumor. A CT-guided biopsy (c) of the enlarging portion of the ablation zone revealed recurrent renal cell carcinoma
Contraindications to Renal Mass Biopsy
Percutaneous biopsy of renal masses is a safe procedure with very low risk to the patient. The only contraindication to renal mass biopsy is coagulopathy that cannot be corrected. For decades, the potential for tract seeding was considered a contraindication to percutaneous biopsy of renal masses; however, recent data suggest that the risk of tract seeding after renal mass biopsy is exceedingly low.
Renal Mass Biopsy Techniques
Coagulation Status
To minimize the risk of hemorrhagic complications from renal biopsy, patients are screened for hemorrhagic diathesis. Screening is warranted even in patients with no history of bleeding. In the United States, most radiologists (84 %) routinely obtain a screening history of potential hemostatic defects and have patients undergo relevant laboratory tests—81 % request prothrombin time, 78 % request partial thromboplastin time, and 59 % request platelet count [43]. At a minimum, a platelet count and international normalized ratio (INR) should be obtained. Patients are advised to stop taking aspirin and other antiplatelet agents 5–7 days prior to the procedure. Patients taking warfarin are asked to stop taking it 5 days prior to the procedure to allow the INR to return to an acceptable range. While anticoagulation agents are suspended, patients may be given heparin or low-molecular-weight heparin to bridge the gap until warfarin can be restarted. Continuous intravenous heparin infusion should be stopped at least 2 h prior to the procedure. Low-molecular-weight heparin (e.g., Lovenox) is suspended only on the day of the biopsy. Although a normal platelet count and normal INR are desirable, we have more liberal guidelines in our practice and perform renal mass biopsy in patients with platelet counts as low as 50,000 and INRs of 1.5.
Imaging
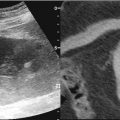
Biopsy of a renal mass can be accomplished using ultrasonography [14, 44–46], CT [14, 29, 38, 44, 47], or MR [38, 48] imaging for guidance. Multiplanar imaging capability and real-time demonstration of the needle tip are major advantages of ultrasound guidance (Fig. 17.17). Because the perinephric fat and the needle are both echogenic, visualization of the needle as it is advanced to the kidney may be difficult under ultrasound guidance, and the utility of ultrasound is limited in obese patients. Also, tumors in the upper pole or medial aspect of the kidney are more difficult to image with ultrasound due to adjacent bowel and intervening lung. Although a nonsedated patient can cooperate with breathing exercises to provide a better window for imaging of the kidney and renal tumors, performing a renal biopsy with breath-hold in deep inspiration is challenging for both the patient and the operator. If patients are sedated during the procedure, it is less likely that they will be able to cooperate with breathing and long breath-hold instructions.
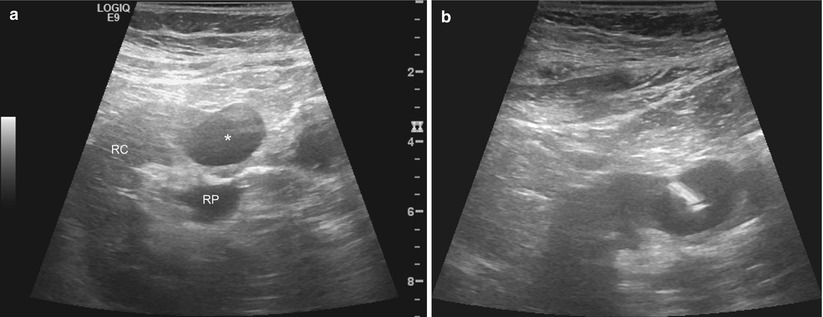

Fig. 17.17
A 53-year-old woman with a history of spindle cell sarcoma of her right iliac bone was found to have a solid mass in the right kidney. A gray-scale sonographic image of the right kidney (a) reveals the mass anterior to the renal pelvis (RP). The mass is hypoechoic compared with the renal cortex (RC). The lesion was targeted under real-time sonographic guidance using the freehand technique. The needle tip is highly echogenic compared to the mass (b)
Alternatively, renal biopsies can be performed under CT guidance. Exophytic renal masses change the contour of the kidney and are easily detected with non-contrast CT (Fig. 17.18). Intraparenchymal tumors without an exophytic component may be more difficult to localize on non-contrast CT images if they are isodense with the kidney. In these cases, administration of iodinated contrast medium immediately prior to the biopsy helps delineate the margins of the tumor (Fig. 17.19). Hypervascular tumors such as RCC show intense arterial enhancement, but nearly all tumors become more hypodense than the normal kidney in the excretory phase of the examination. As the normal kidney continues to accumulate and excrete iodinated contrast medium, the difference in density remains perceptible for the duration of the biopsy, allowing targeting of the tumor. Anatomic structures such as intervening bowel and pleural space that may be hard to visualize on ultrasonography are readily visible on CT. A major drawback of CT guidance for renal mass biopsy is the intermittent nature of imaging in a single axial plane. It may be difficult to image the needle and the tumor in the same plane, particularly in cases of small tumors. Real-time CT fluoroscopy can alleviate some of these challenges but can also increase radiation dose to the patient and the operator. Ultrasonography and CT guidance can be combined to optimize targeting of renal tumors in difficult cases.
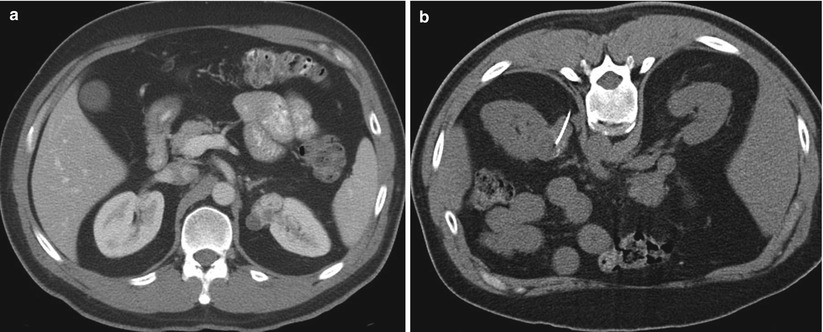
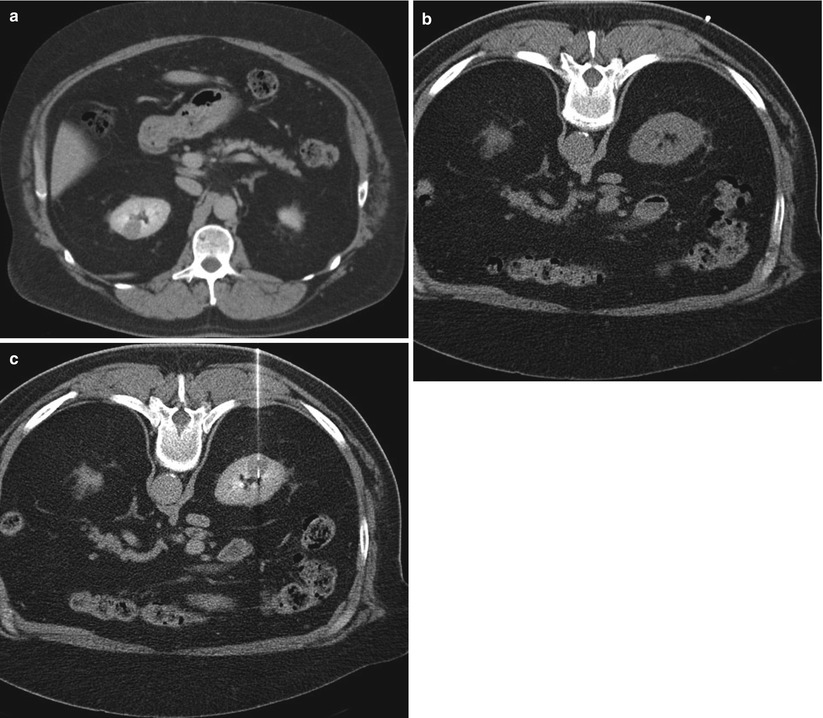

Fig. 17.18
A 42-year-old man with a renal mass was referred for biopsy. A diagnostic CT image (a) reveals a hypervascular, exophytic mass that alters the smooth contour of the left kidney. A biopsy was performed under CT guidance (b). There was no need for administration of contrast medium because of the exophytic nature of the mass. An out-of-plane technique was used to minimize the risk of pneumothorax. A pathologic evaluation revealed renal cell carcinoma

Fig. 17.19
A 68-year-old man with coronary artery disease was referred for biopsy of a right renal mass that was detected incidentally. A diagnostic CT image (a) in the late phase of contrast enhancement reveals a relatively low-density solid mass (1.6 cm diameter) that is confined to the renal parenchyma and does not change the contour of the kidney. An axial non-contrast CT image of the right kidney in the prone position (b) does not reveal the tumor. After administration of contrast medium, the lesion became conspicuous and was targeted for biopsy (c). A pathologic evaluation revealed oncocytoma
Renal tumors are particularly well suited for biopsy under MRI guidance if an interventional MRI suite is available. Renal tumors often demonstrate high signal intensity on T2-weighted images, allowing easy detection. Any number of imaging planes—such as axial, oblique axial, sagittal, or sagittal oblique—can be used to target the tumor using intermittent or real-time imaging (Fig. 17.20). MRI-compatible needles, constructed of nonferromagnetic alloys, are available for use within the magnetic field. There are no published reports directly comparing the diagnostic success rates of the different imaging modalities used in image-guided renal mass biopsy. Anecdotal reports indicate that diagnostic yield, sensitivity, or negative predictive value for image-guided renal mass biopsy is independent of the imaging modality [14, 49]. Most important, the imaging features of an individual mass along with the experience and personal preference of the operator help determine the most useful method of tumor localization and targeting.
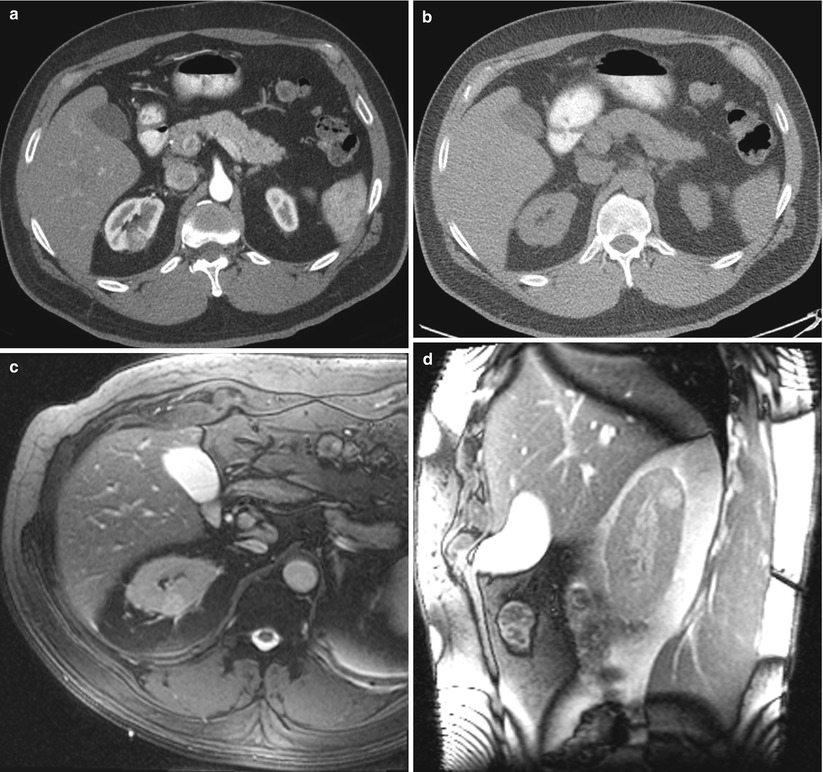
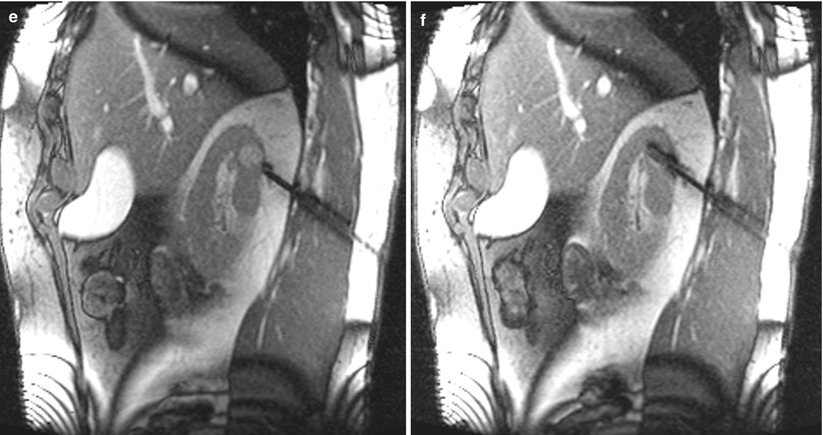


Fig. 17.20
A 56-year-old man was found to have a 1.6-cm mass in his right kidney. The mass was discovered incidentally when a CT scan was performed for a non-urological complaint. Because of his other comorbidities, he was referred for biopsy to determine the need for surgery or other interventions. An axial CT image of the abdomen after administration of contrast material (a) reveals a hypervascular mass in the posterior upper pole of the right kidney. The mass could not be visualized on non-contrast CT images (b). An axial trueFISP MRI of the abdomen (c) in the prone position reveals a hyperdense tumor in the right kidney. This image also reveals the lung base interposed between the tumor and the chest wall. Sagittal MR images reveal a biopsy trajectory that avoids the lung base (d–f). A biopsy revealed oncocytoma. The patient did not require treatment
Devices
Renal masses can be sampled both by fine-needle aspiration (FNA) and by tissue-cutting tip needles. FNA samples are obtained using 21-gauge or smaller needles, which are believed to minimize blood contamination of the sample and to yield a larger number of cells. Larger core biopsy samples are obtained using automated “biopsy guns” that are equipped with tissue-cutting tip needles. Both FNA and core biopsies can be performed through a coaxial guide or cannula [50]. Once the guiding cannula is advanced to the surface of the tumor, multiple samples can be obtained without reinserting the sampling needle through a new tract (Fig. 17.21). This technique decreases patient discomfort and shortens the procedure time. The coaxial technique helps avoid repeated contact of a needle that may be contaminated with tumor cells with the overlying abdominal or retroperitoneal tissues, thus reducing the potential risk of tract seeding. In addition, procoagulants or an absorbable gelatin sponge (Gelfoam) can be injected into the tract to promote hemostasis at the end of the procedure [49]. For most renal biopsies, an 18- to 16-gauge guide needle is used. With this approach, 22-gauge needles can be used in FNA biopsy, and 20- to 18-gauge cutting needles can be used in core biopsy.
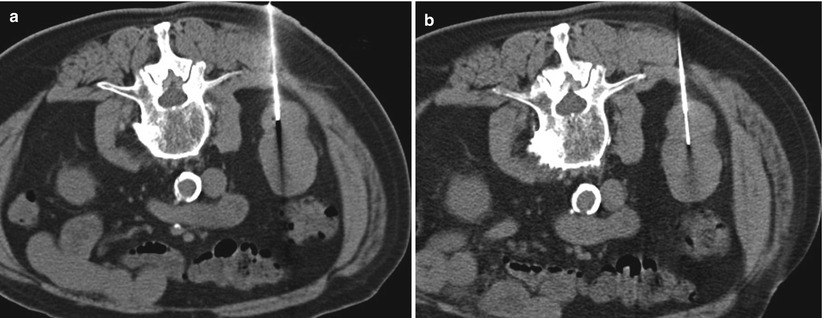

Fig. 17.21
An axial CT image of the abdomen (a) reveals a guide needle placed in an exophytic mass along the posterior aspect of the right kidney. (b) A smaller gauge biopsy (FNA or core) can be advanced in coaxial fashion to obtain a biopsy sample. As long as the guide needle remains in place, the biopsy needle can be reinserted to obtain additional samples
Approach and Relevant Anatomy
Most renal mass biopsies are performed with the patient in a prone (Fig. 17.22), semiprone, or decubitus position. During ultrasound-guided biopsies, the patient can be rotated to an arbitrary oblique position to allow a better window to visualize the tumor or a safer tract for advancing the needle. For CT-guided procedures, an ipsilateral decubitus position helps decrease the volume of the dependent thoracic cavity, thus reducing the lung excursion in the caudal direction (Fig. 17.23). This position provides a safer approach to upper pole tumors and has a lower risk of pneumothorax (Fig. 17.24). Otherwise, the CT gantry may be tilted to allow an oblique axial plane of imaging to avoid traversing the lung base (Fig. 17.25). Occasionally, a large renal mass in a very thin patient can be approached laterally (Fig. 17.26) or anteriorly (Fig. 17.27) with the patient in the supine position. Small tumors in the anterior aspect of the kidney may warrant a lateral approach with the patient in the supine or prone position (Fig. 17.28). If an intercostal approach is needed, care must be taken to avoid injury to the neurovascular bundle that is often located along the inferior margin of the ribs. Transhepatic and transsplenic biopsy of renal masses may be considered if no other needle paths are available, but these approaches are rarely used (Fig. 17.29). Ideally, different areas of the tumor should be targeted to obtain a representative sample. Necrotic areas, usually encountered in the center of large tumors, should be avoided [51]. For tumors smaller than 4 cm, one central and one peripheral core biopsy is recommended, whereas for tumors larger than 4 cm with a potentially necrotic center, two peripheral core biopsies are recommended [51]. Core biopsy samples should be visually inspected to estimate their size and quality. Fragmented or small samples (<1 cm) warrant additional sampling of the tumor [32].
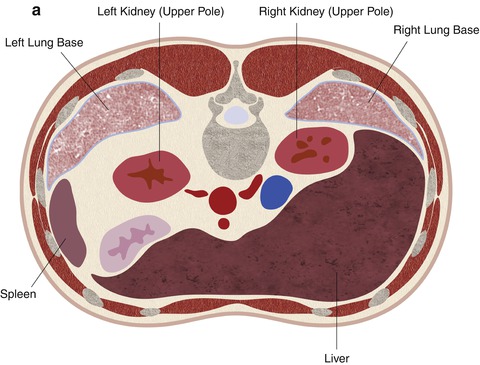
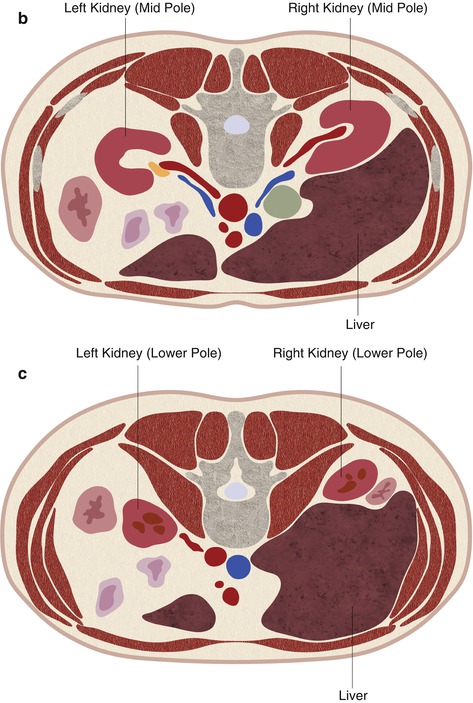
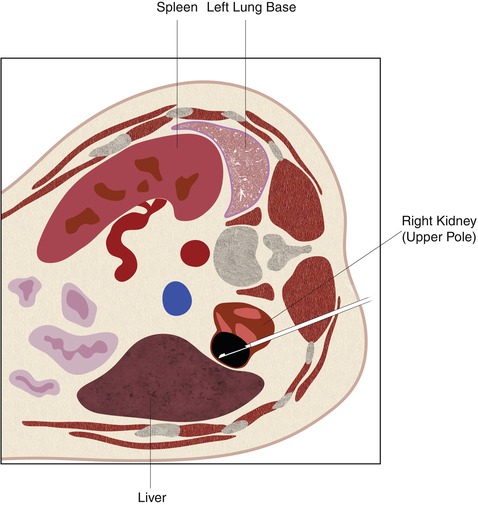
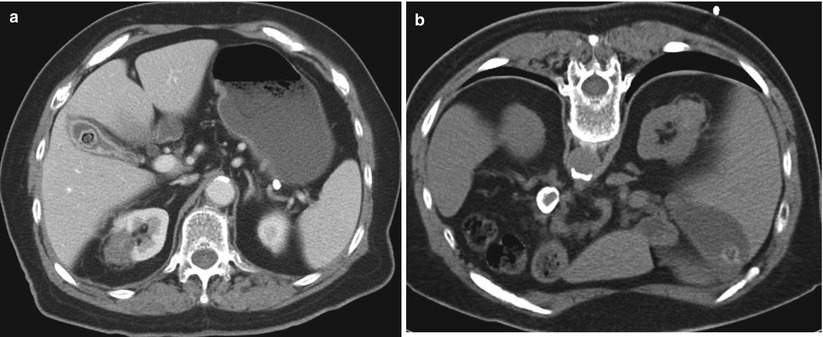
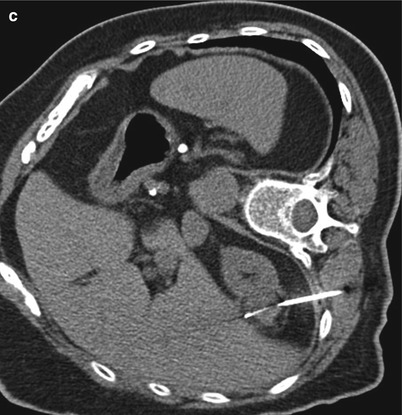
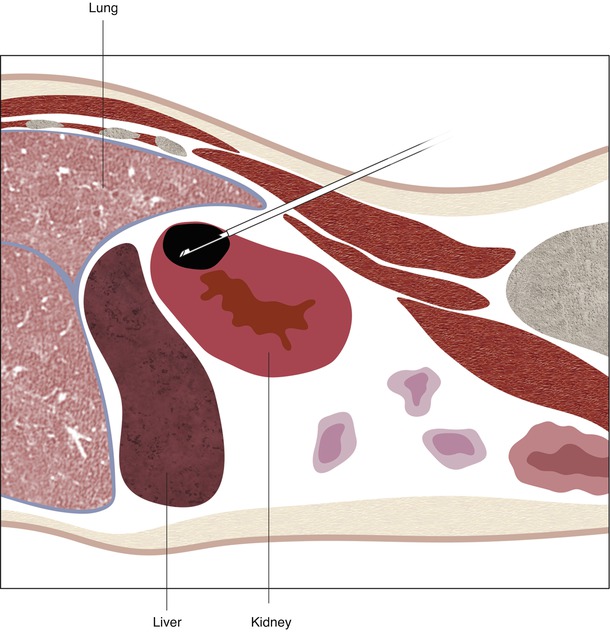
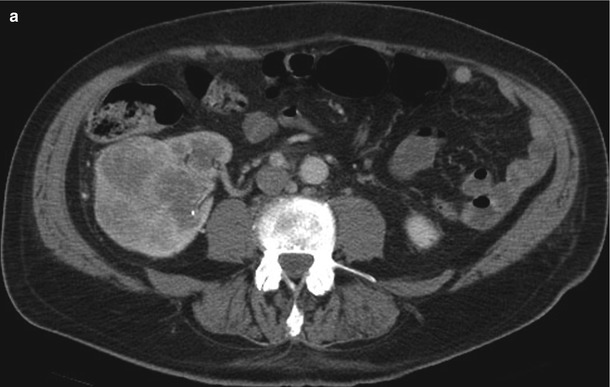
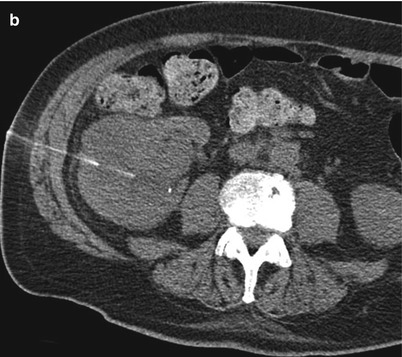
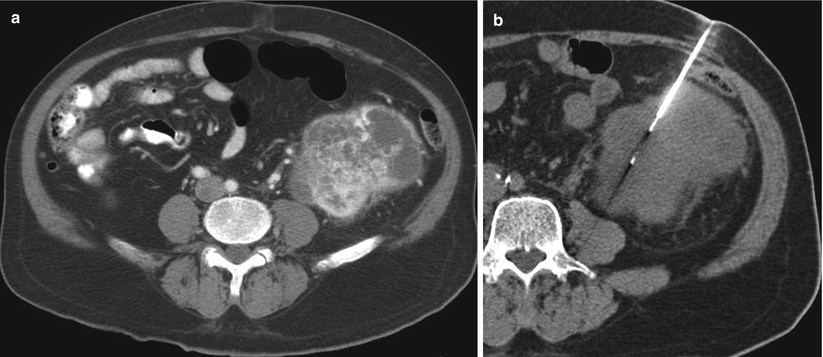
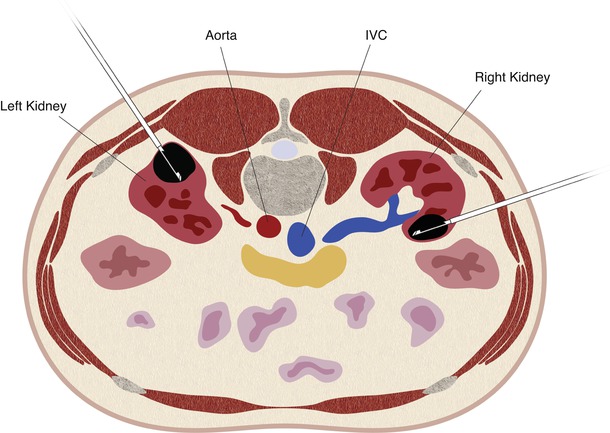
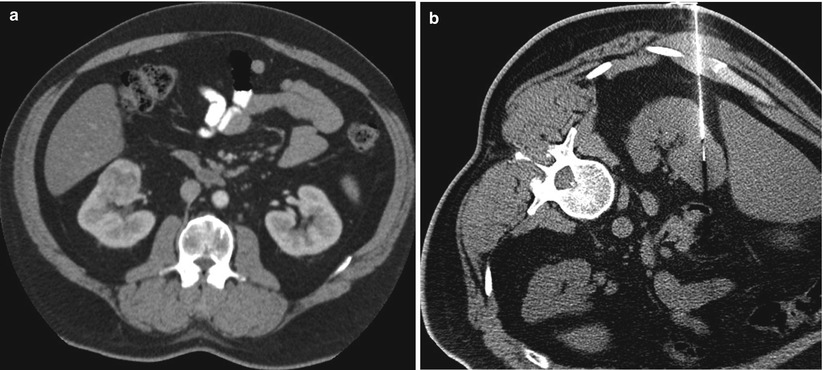


Fig. 17.22
A schematic demonstration of the relevant anatomy with the patient in the prone position. In the prone position, the lung bases are often interposed between the chest wall and the upper pole of the kidneys (a). The mid pole (b) and lower pole (c) of the kidneys are usually surrounded by retroperitoneal fat

Fig. 17.23
When the patient is placed in an ipsilateral decubitus position, the ipsilateral lung is compressed and the lung base is no longer interposed between the upper pole of the kidney and the chest wall. This will decrease the risk of pneumothorax during CT-guided biopsy of tumors in the upper pole of the kidney


Fig. 17.24
A 77-year-old woman with a history of renal cell carcinoma underwent percutaneous radiofrequency ablation. Follow-up images were suspicious for recurrence. The patient was referred for biopsy. An axial CT image of the abdomen (a) reveals the zone of ablation in the lateral aspect of the upper pole of the right kidney. The patient was placed in the prone position for biopsy. An axial CT image of the abdomen (b) reveals interposition of the lung parenchyma between the kidney and the intended needle insertion site. The patient was then placed in the right lateral decubitus position. In this position (c), the right lung is compressed, the volume of the right hemithorax is decreased, and the lung is no longer in the path of the needle. Core biopsies of the ablation zone revealed necrotic tissue and no viable tumor

Fig. 17.25
A schematic illustration of the approach to upper pole tumors, avoiding the lung base. The CT gantry can be tilted to outline the path of the needle. Alternatively, an out-of-plane approach can be achieved by triangulation. In this case, axial CT images will reveal only a segment of the needle. Using MRI, the entire biopsy can be planned and carried out in the sagittal plane


Fig. 17.26
A 67-year-old man was referred for biopsy of a large right renal mass. An axial CT image of the abdomen reveals a large exophytic mass arising from the right kidney (a). A CT-guided biopsy was performed in the supine position, using a lateral approach (b). Only fine-needle aspiration biopsy samples were obtained. A pathologic evaluation revealed high-grade carcinoma but could not distinguish between renal cell carcinoma and urothelial carcinoma. Core biopsy samples may have been beneficial for establishing a firm diagnosis

Fig. 17.27
A 72-year-old man with a large left renal mass was referred for biopsy. An axial CT image of the lower abdomen (a) reveals a large hypervascular mass arising from the left kidney, with areas of necrosis. A CT-guided biopsy (b) was performed from an anterior approach, with the patient in the supine position. A fine-needle aspiration biopsy was performed, and an immediate assessment of the slides revealed viable tumor cells. Core biopsy samples were obtained from the same region. The final cytopathology report was “rare atypical cell clusters, suspicious for renal cell carcinoma.” A histological evaluation report of the core biopsy sample revealed renal cell carcinoma, clear cell type, and Furman’s nuclear grade 3

Fig. 17.28
A schematic illustration of a lateral approach for an anteriorly located renal tumor (right kidney) compared with a posterior approach for a posteriorly located renal tumor (left kidney)

Fig. 17.29
A 57-year-old man was referred for biopsy of a right renal tumor. An axial CT image of the abdomen in the supine position reveals a solid enhancing mass in the anterior aspect of the right kidney (a). A CT-guided biopsy was performed in the lateral decubitus position, with a lateral approach (b). This position helped displace the liver anteriorly and provided a safe path for the biopsy needle
Potential Complications
Some of the largest recent series of renal mass biopsy have reported very few or no major complications [14, 32, 44, 45, 52–54]. In a total of 362 biopsies, there were 17 minor complications (4.7 %) and 1 major complication (0.3 %). Potential complications for renal mass biopsy include perinephric hemorrhage, hematuria, arteriovenous fistula, infection, pneumothorax, and needle tract seeding.
Most renal tumors, particularly RCCs, are hypervascular and such are prone to bleeding after percutaneous biopsy. Small perinephric or subcapsular hematomas accompany these procedures in 44–91 % of cases but are often self-limited, do not cause any symptoms, and do not require additional intervention (Fig. 17.30) [47, 55]. Renal hemorrhage that necessitates hospital admission or blood transfusion occurs in 1–2 % of cases [33, 56]. Subcapsular hematomas are often asymptomatic (Fig. 17.31), but they can be large and may cause significant pain and severe hypertension [57]. Hematuria as a result of renal mass biopsy is also uncommon, but it may lead to ureteral obstruction and pain [58]. An arteriovenous fistula should be considered in cases of persistent bleeding [56, 59]. If careful attention is given to aseptic technique, the risk of infection is minimal. With the patient in the prone position, the posterior sulcus extends over the upper pole of the kidney in most patients. During CT-guided renal mass biopsy, transgression of the pleural space and/or lung may cause a pneumothorax [60, 61]. Placing the patient in an ipsilateral decubitus position or using a triangulation technique with subcostal needle insertion helps reduce the risk of pneumothorax [62].
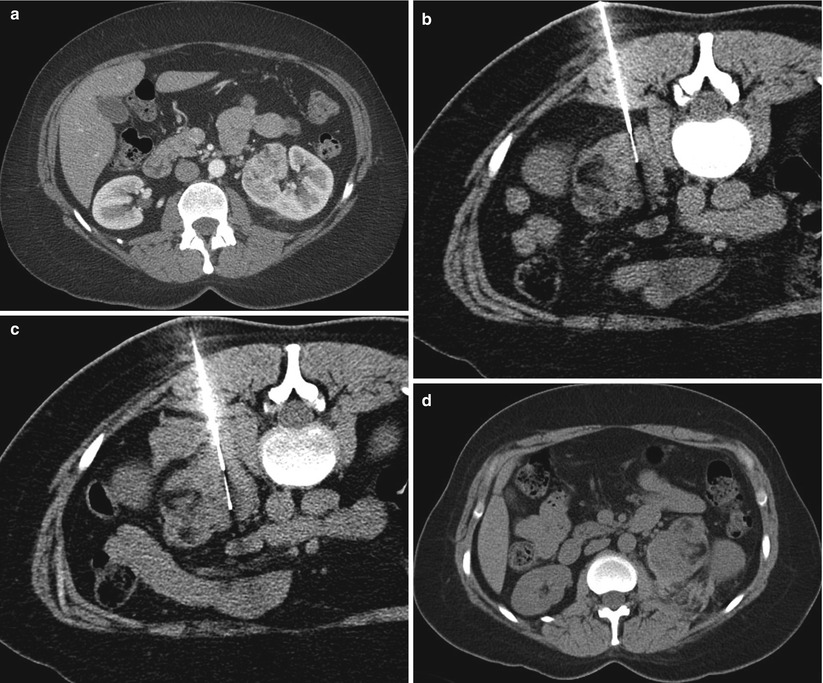
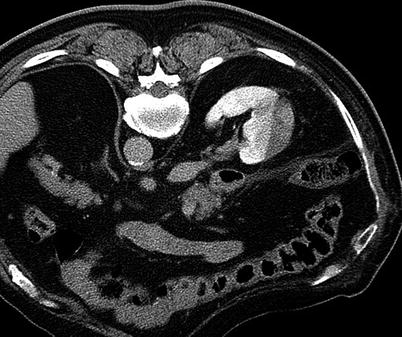

Fig. 17.30
A 47-year-old woman with a history of synovial sarcoma was referred for biopsy of a left renal mass. A diagnostic axial CT image of the abdomen (a) reveals a hypervascular mass in the medial aspect of the left kidney. A CT-guided biopsy (b) was performed in the prone position. Shortly after the first biopsy sample was obtained, a CT image (c) revealed development of a moderate-sized retroperitoneal hematoma. An axial CT image of the abdomen (d) 4 h later revealed no change in the size of the retroperitoneal hematoma. The patient was hemodynamically stable and was discharged home with no other interventions

Fig. 17.31
A subcapsular hematoma after a renal mass biopsy is often self-limited and has no major consequences. If the hematoma enlarges sufficiently, it can cause pain or hypertension
For many years, percutaneous renal mass biopsy was considered inappropriate because of fear of needle tract seeding. Although occasional cases of tract seeding have been reported after percutaneous biopsy of renal tumors, the actual incidence is low. The overall estimated risk of needle tract seeding in urologic malignancies is less than 0.01 % [63], which is comparable to risk of this complication in other abdominal biopsies. In recent series of renal tumor biopsy, no case of needle tract seeding was reported [32, 33, 47, 52, 59, 64–69]. The lack of tract seeding in recent series may be related to the widespread use of the guiding cannula and coaxial technique during image-guided biopsy of renal tumors. It has been reported that urothelial carcinomas are at higher risk of seeding than are RCCs [63, 70], and some researchers have recommended that an infiltrative renal mass should not be biopsied unless lymphoma is a primary consideration [53]. However, we have found very little scientific evidence for this recommendation [70].
Management of Complications
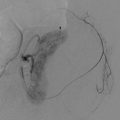
Large or persistent perinephric hemorrhage may cause a drop in hemoglobin levels and may cause symptoms related to blood loss. These symptoms often include one or more of the following: pain, hypotension, tachycardia, diaphoresis, weakness, and drowsiness. Transfusion of packed red blood cells may be warranted if the patient is symptomatic. If the bleeding persists, an angiogram and possible selective embolization may be warranted (Fig. 17.32) [71]. Gross hematuria, if self-limited and asymptomatic, does not require intervention. However, ureteral obstruction may require placement of a stent, preferably retrograde via cystoscopy rather than antegrade via percutaneous nephrostomy. Persistent hematuria should be evaluated by angiography to rule out a pseudoaneurysm or arteriovenous fistula. If the course of the needle is close to the base of the lung, observation and chest radiography are warranted after the biopsy. A small pneumothorax may be observed, but an enlarging pneumothorax may require chest tube placement.
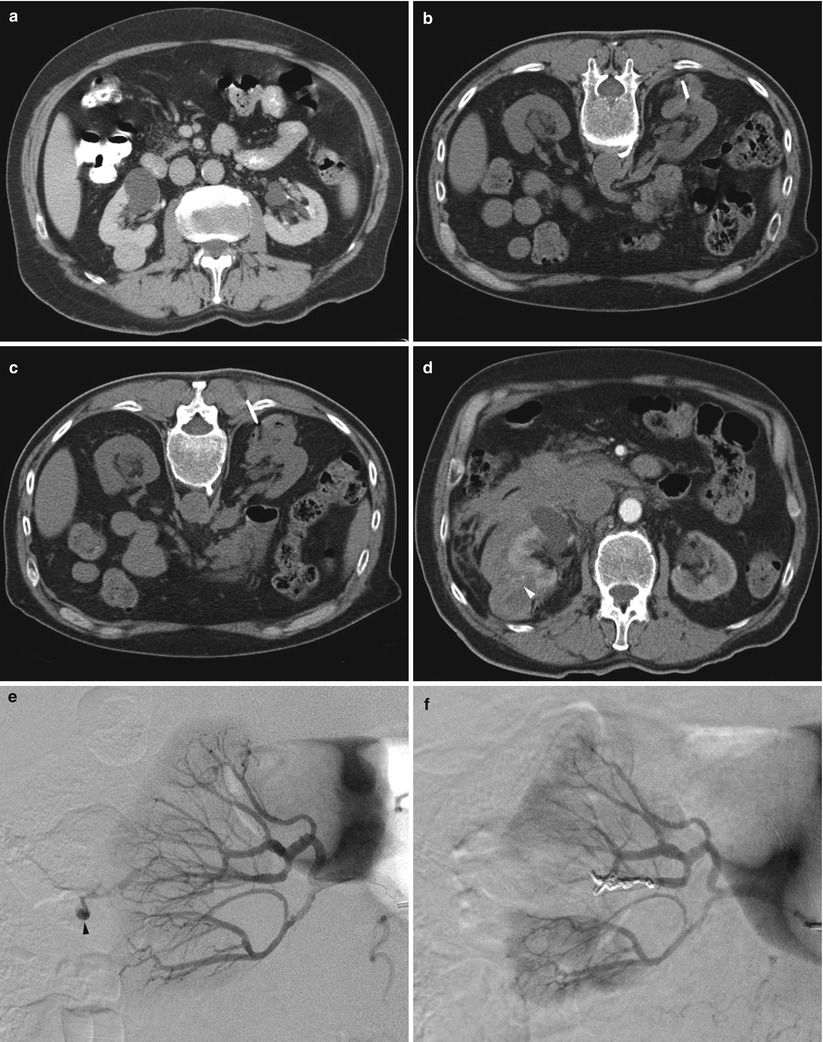

Fig. 17.32
A 79-year-old man with a right renal mass was referred for biopsy to determine the need for surgery or other interventions. A diagnostic CT image of the abdomen reveals a solid enhancing mass in the posterior aspect of the right kidney (a). A CT-guided biopsy was performed in the prone position (b). During the procedure, the patient developed a hematoma surrounding the tumor (c). A contrast-enhanced CT scan was performed because of increasing flank pain during the recovery period (d). The image revealed a larger retroperitoneal hematoma, with evidence of active contrast extravasation (arrowhead in d). A selective right renal artery angiogram (e) reveals active bleeding (arrowhead). After embolization of the bleeding artery, an angiogram (f) revealed no further extravasation
Outcomes and Results of Renal Mass Biopsy
In studies published prior to 2001, renal mass biopsy yielded an accurate diagnosis in 88.9 % of 2,474 biopsies for suspected RCC [6] (Table 17.2). More recently, renal mass biopsy has received increasing attention and has played a substantial role in the management of patients with renal tumors. Various studies have been published reporting the success rates and accuracy of these biopsies, albeit in an inconsistent manner. There are variations in technique. Several studies reported only core needle biopsy, whereas others reported the results of FNA biopsies, and still others reported success rates for both FNA and core needle biopsies. Most studies used CT and/or ultrasound to guide needle placement, but definitions of nondiagnostic, inadequate, indeterminate, and failed biopsy varied between studies. Nevertheless, the reported rate of technical failure due to insufficient material has dropped from 9 % before 2001 to approximately 4 % in the more recent series [6] (Table 17.3). The rate of indeterminate or inaccurate pathologic findings was reported to be 10 % in earlier studies, but this rate has decreased to 4 % in the more recent series [6]. Given these findings, when initial biopsy results are inconclusive, a repeat biopsy is warranted. A definitive diagnosis can be obtained in 83–100 % of repeat renal mass biopsies [33, 47, 49, 64, 65, 69, 72, 73].
Table 17.2




Studies published before 2001 of renal mass biopsy of solid renal tumors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree