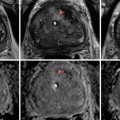
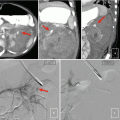
Fig. 18.1
An example of sampling error from a renal biopsy. (a) The tumor on contrast-enhanced imaging (white arrow). (b) The needle trajectory and biopsy performed from an outside hospital which showed normal parenchyma. (c) Repeat biopsy performed directly into the lesion between rib 11 and 12 which later demonstrated a Fuhrman grade III clear-cell kidney tumor
Diagnosis of Histologic Subtype and Grade
Percutaneous renal biopsy may not only serve to diagnose renal cell carcinoma, but may provide further pathologic information including the identification of subtype and assessment of tumor grade. If a high-grade tumor or an aggressive subtype is found, a different management strategy may be worth considering. The diagnostic accuracy of distinguishing subtype of malignant tumors is approximately 80 % [12]. A study by Renshaw et al. assessed the accuracy of fine-needle aspiration (FNA) in identifying or distinguishing subtypes of renal cell carcinoma. There were 34 tumors studied, and of this, 74 % were correctly subtyped when compared to the nephrectomy specimen [13]. The accuracy of distinguishing grade and histology may be further improved with core needle biopsy. Neuzillet et al. assessed 88 patients who underwent a CT-guided core needle biopsy and found it accurate in identifying subtype and Fuhrman grade in 92 % and 69.8 %, respectively [14]. Incorporation of adjunct pathologic methods such as immunohistochemistry and assessment of cytogenetic changes may further improve the capability to characterize tumor subtype.
Biopsy Indications
Established Indications
There is no question that percutaneous renal biopsy will continue to expand and play a role in the diagnosis and management of renal masses. Currently, most experts recommend biopsy of renal masses when the diagnosis would change management, including the choice of surgery or systemic therapy. Established indications include patients with a renal mass who have an advanced, nonrenal malignancy such as melanoma, lung cancer, breast cancer, lymphoma, and colon cancer. Often, imaging may be suggestive of a metastasis when enhancement is less robust than for primary renal tumors; however, this characteristics is not definitive [15]. If the renal mass detected is a metastasis, the management is much different except in rare cases where there is a role for metastasectomy for an isolated lesion. In addition, patients with a renal mass that is deemed technically unresectable or who may not be a surgical candidate, a biopsy can help guide selection for systemic therapy. In patients who have a history of urinary tract infections such as pyelonephritis, a renal lesion may represent a focal abscess or carbuncle. In this setting, a percutaneous renal biopsy may avoid an unnecessary surgery. Finally, in patients with an atrophic kidney, it is not uncommon to have a spared region of parenchyma that may also resemble a tumor (a pseudotumor). It is often not possible to give patients intravenous contrast in this situation due to chronic kidney disease, and we prefer a confirmatory biopsy prior to offering management (Fig. 18.2a–c).
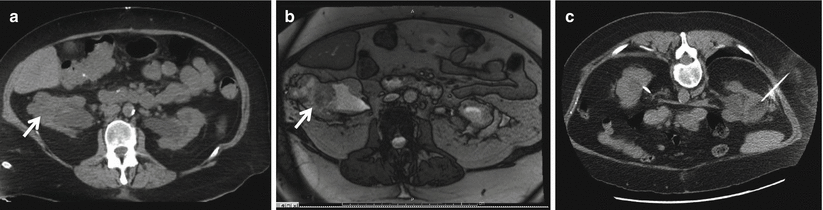

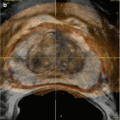
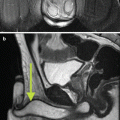
Fig. 18.2
Non-contrast (a) CT and (b) MRI in a 62-year-old female with CKD stage IV who had an irregular 4.5 cm right renal lesion (white arrow). (c) A percutaneous biopsy was performed for confirmation that this represented a mass versus asymmetric cortical hypertrophy. The biopsy confirmed the presence of a Fuhrman grade II clear-cell kidney tumor
Emerging Indications
With improvements in detection of benign lesions and detection of subtype, there are emerging indications for biopsy. We consider a renal mass biopsy in elderly patients or those with small renal masses where identification of an oncocytoma or oncocytic tumor with a more indolent potential would lead to consideration of nonoperative management. Additionally, prior to enrollment in an active surveillance protocol, we believe identification of tumor characteristics provides insight into tumor biology. Occasionally, we identify an aggressive subtype or a high-grade lesion and consider treatment for these individuals. For patients who are considered for laparoscopic or percutaneous ablation, many advocate for percutaneous biopsy before or at the time of treatment [4]. While both approaches are acceptable, we generally perform this in a separate setting prior to treatment and then proceed with ablation once a malignant histology is confirmed. We advocate combing procedures in certain circumstances such as when stopping anticoagulants is an issue. Finally, in cases of an extremely challenging partial nephrectomy where resection of the tumor will require collecting system entry and enucleation of the renal hilum, we occasionally utilize percutaneous biology to better characterize the lesion. In Fig. 18.3a–d, we demonstrate one such case in a lesion with a high nephrometry score (11) where three regions of the tumor were sampled and a Fuhrman grade II clear-cell RCC was identified. Due to the perceived lower grade and biologic potential, we did an open partial nephrectomy utilizing enucleative resection. Had this been a very high-grade lesion or an aggressive histologic type, we had discussed proceeding with a laparoscopic radical nephrectomy.
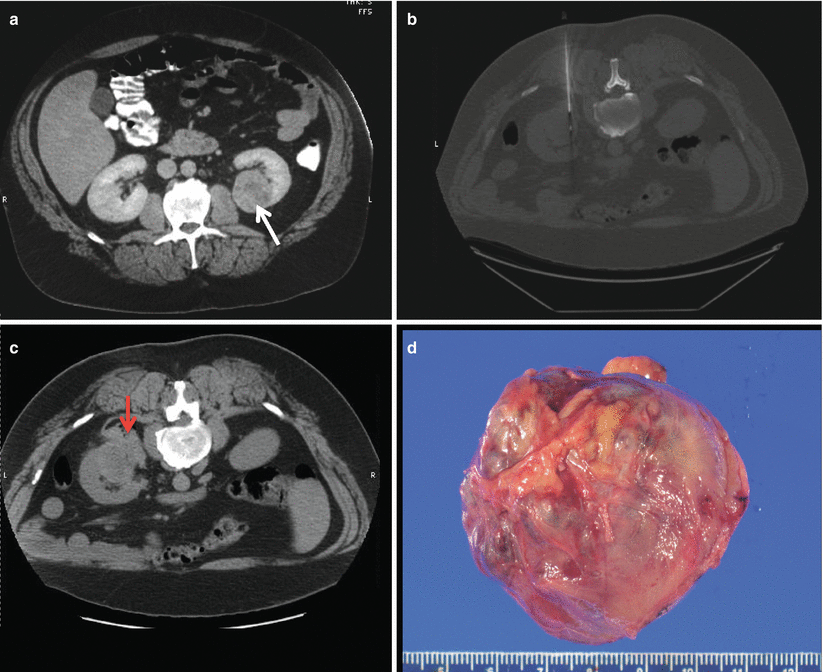

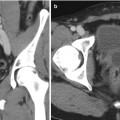
Fig. 18.3
(a) A 47-year-old man with an incidentally detected 5.5 cm left renal mass with a nephrometry score of 11 ph (white arrow). (b) A renal biopsy was performed to help guide the choice of therapy. (c) There was some mild asymptomatic hematoma (red arrow) after the procedure. (d) Once several cores demonstrated a Fuhrman grade 1–2 clear-cell renal mass, we proceeded with a complex partial nephrectomy confirming the presence of a low-grade T1b renal tumor
Complications of Renal Mass Biopsy
The initial experience with renal mass biopsy was associated with substantial morbidity. Recently, reviews have demonstrated that mortality is exceedingly rare and even minor complications are <2 % [9]. Complications of renal mass biopsy include tumor seeding, bleeding, infection, arteriovenous fistula, and pneumothorax. Each of these risks will be individually explored in the following sections. In light of the low rate of complications, the benefit of avoiding a morbid operation often outweighs the risk associated with renal mass biopsy.
Bleeding Risk
Hemorrhage is the most frequently encountered complication of renal biopsy. Modifiable factors such as coagulation parameters and hypertension should be optimized; however, even when these factors are controlled, there is still a high bleeding risk. It is recommended that patients on blood thinners be evaluated for cessation prior to biopsy, and those patients with history of bleeding diathesis be properly worked up. It has been widely believed that needle size plays a role in the risk of bleeding. For native kidneys, it has been demonstrated that the risk of bleeding can be fourfold greater if a 14 gauge needle is used versus smaller gauges [16]. However, in animal models, Gazelle and colleagues found no significant difference in bleeding risk between 18, 20, and 22 gauge needles [17]. We prefer to use a 17 gauge introducer with an 18 gauge needle to provide quality tissue cores with the theoretical benefit at limiting the risk of bleeding.
Identification of bleeding after biopsy is highly dependent on the use of post-procedural imaging. Ralls and colleagues investigated the incidence of bleeding by performing a CT scan and ultrasound 24–72 h after biopsy. CT scan was more sensitive and specific than US for detecting hemorrhage. A surprisingly high frequency of patients (90.8 %) were identified with a hemorrhage on CT scan. However, only two of these hematomas were clinically significant requiring treatment. While the risk of bleeding is high, generally, most patients will have a small hematoma that is not clinically significant.
Post-biopsy patients must be observed while lying supine to tamponade any bleeding. The optimal timing of observation after a renal mass biopsy has not been established. In the native renal biopsy literature, the timing of complications has been specifically addressed. Historically, patients were observed for 24 h for observation. Two large series assess the timing of major complications in patients who were kept for overnight observation. Several studies have suggested that 42–52 % and 85–95 % of complications are observed within 4 and 12 h, respectively [18, 19]. Most centers observe patients undergoing renal mass biopsies for approximately 4 h unless they are considered to have a significant risk of bleeding.
Significant bleeding complications appear to be higher in patients who need to undergo a random kidney biopsy for diagnosis of medical renal disease. Many of these patients may have platelet dysfunction from uremia or from aspirin or clopidogrel, thrombocytopenia, as well as having significant hypertension. A large meta-analysis was found; the overall rate of transfusion and gross hematuria is 1 % and 3.5 %, respectively, [16] much higher than what is observed in modern series of renal mass biopsies. Coprai and colleagues found that having elevated creatinine or being anemic are risk factors for post-biopsy hemorrhage with random kidney biopsies [16], which are obviously less frequent in patients who undergo a renal mass biopsy.
Occasionally, patients may develop hemodynamic instability for significant bleeding. In our experience, this is more common in frail patients or those on multiple hypertensive medications who have remained NPO for several hours before the procedure (Fig. 18.4a, b). Wood and colleagues examined clinically significant bleeding complications (requiring blood transfusion or embolization) after 79 renal mass biopsies. In this study, there were no major bleeding complications. In the case of a clinically significant bleed, rarely is surgery required. Conservative management with fluids and/or transfusion is generally all that is necessary. In the setting of a major post-biopsy hemorrhage, if angiography is available, we advocate for immediate selective embolization. While bleeding almost always stops with conservative management, embolization of active bleeding may prevent expansion into a large hematoma that could take months to reabsorb and cause a significant delay in surgical management.
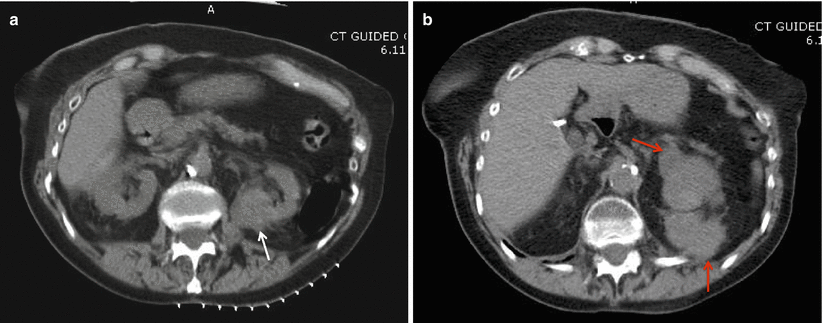

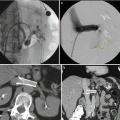
Fig. 18.4
(a) A posterior 3.8 cm renal lesion (white arrow) in an 86-year-old female. (b) After biopsy of the lesion, there was significant bleeding anterior and posterior to the lesion (red arrows). The patient was managed with admission and conservative measures
Risk of Seeding
The risk of needle track seeding of tumor cells is a concern in several solid nonrenal malignancies. In the kidney cancer literature, the risk is exceedingly low. Herts and colleagues estimate the risk of tumor seeding to be 0.01 % [20]. A study by Neuzillet et al. explored this risk in a series of 88 patients and found no reports of tumor seeding after biopsy [14]. Many believe the use of a coaxial sheath to pass the biopsy needle through for kidney biopsy could limit the risk of seeding. Additionally, there has been speculation that a large needle increases the risk of seeding [21]; however, no definitive evidence exists. This is the case for kidney tumors [22].
In the last 20 years, there is only one reported case of seeding in the literature. In this case, there was reported seeding of the biopsy tract after an ultrasound-guided biopsy of a Bosniak IIF renal cyst. In this report, there were four needle passes during FNA in addition to two core biopsies without the use of a coaxial sheath. On final pathology, the authors believed there was extrarenal extension of a 6.5 cm high-grade papillary tumor believed along one of the needle tracks [23]. This unique case occurred with a cystic lesion, that many centers would generally exclude from biopsy given the higher-risk cyst rupture and the potential for tumor spillage [24]. Additionally, while the authors believed the spread was along needle tracks, there is no definitive way to confirm the path of a biopsy needle ex vivo.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree