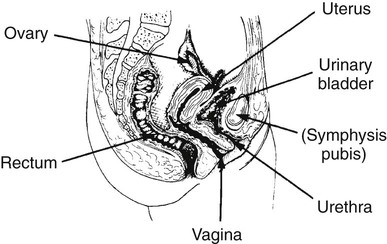
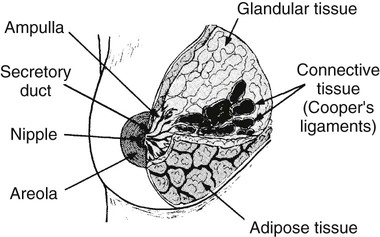
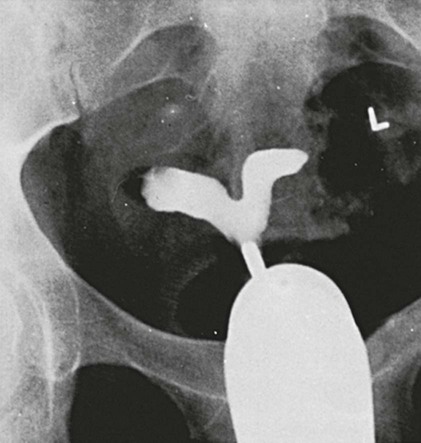
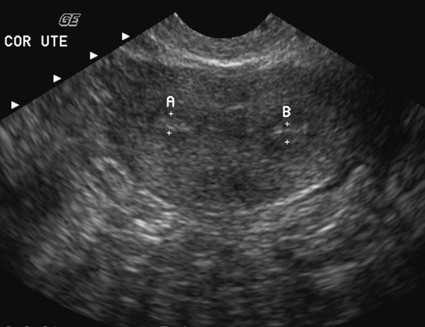
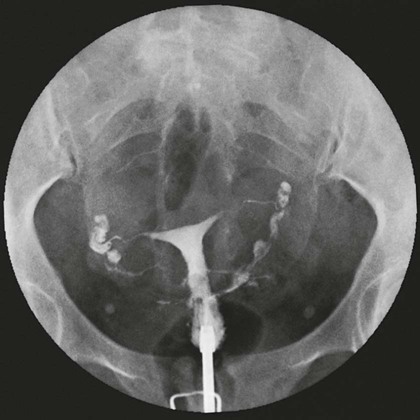
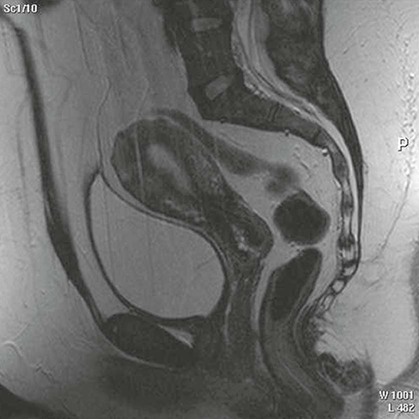
On completion of Chapter 10, the reader should be able to: • Discuss the basic anatomic structures associated with the male and female reproductive systems. • Briefly explain the role of general radiography, mammography, diagnostic medical sonography, computed tomography, and magnetic resonance imaging in the diagnosis and treatment of reproductive system disorders. • Compare and contrast breast imaging modalities, including diagnostic versus screening mammography, localization techniques, and sonography. • Differentiate among the major congenital anomalies of the female reproductive system. • Describe the various neoplastic diseases of both the female and male reproductive systems in terms of etiology, incidence, signs and symptoms, treatment, and prognosis. • Differentiate among the common disorders during pregnancy and explain the role of diagnostic medical sonography in the management of the gravid female. Female Reproductive System The female reproductive system consists of one pair of ovaries, which are the primary sex organs, and the secondary sex organs, which include one pair of fallopian tubes, the uterus, the vagina, and two breasts (Fig. 10-1). The primary function of the system is to provide a female reproductive cell (the ovum), hormones, and a site for the development of the zygote. Occasionally, lack of proper uterine support is present, and a device known as the pessary (Fig. 10-2) is inserted into the vagina to provide proper support. Current research has demonstrated that the majority of women who use the pessary are very satisfied, as the device relieves the majority of urinary symptoms. Some women report that although the use of a pessary helps improve urinary symptoms, stress incontinence remains an ongoing problem. However, the use of the pessary continues to be the treatment for pelvic organ prolapse and may assist with the quality of life for many of these patients. Anatomically, breasts are attached via Cooper ligaments to the pectoral muscles, and this gives the breast its contour and shape. The breast consists of about 12 lobes separated by connective tissue, much like the spokes of a wheel. The lobes are further divided into lobules clustered around small ducts. These small ducts join to form larger ducts, which terminate at the nipple (Fig. 10-3). Breasts function as accessory reproductive glands to secrete milk for the newborn. During pregnancy, changes in estrogen and progesterone levels prepare breasts for lactation. Approximately 3 days after delivery, a lactogenic hormone stimulates the secretion of milk. One of the most common radiographic studies of the female reproductive system is the hysterosalpingography (HSG). It is an examination performed for screening of the nongravid (nonpregnant) woman, especially in cases of suspected infertility. A common finding in cases of infertility is nonpatent fallopian tubes. In addition, although HSG does not define the extent of certain conditions such as endometriosis, it is useful in revealing the shape of the uterus and certain characteristics of the fallopian tubes other than their patency. The practice guideline of the American College of Radiology (ACR) for the performance of HSG is to inject approximately 10 to 30 mL of contrast medium into the uterine cavity and the injection should be done slowly to avoid causing spasms and discomfort. Spillage of the contrast medium from the fallopian tubes indicates the patency of the tubes (Fig. 10-4). Typically, HSG is used for diagnostic purposes, but it can also be used therapeutically for restoring tubal patency or to dilate or stretch the fallopian tubes. Magnetic resonance imaging (MRI) is now often used in conjunction with sonography in the evaluation of the female pelvis (Figs. 10-5 and 10-6). MRI, like sonography, uses no ionizing radiation and is noninvasive. MRI gives detailed information on pelvic, uterine, and ovarian masses (Fig. 10-7). In cases of ovarian cancer, MRI accurately demonstrates proliferation into other pelvic structures. In addition, multiple leiomyomas can be detected and localized in a short period. MRI is increasingly being used to assist in differentiating between malignant and benign solid lesions within the breast. Fat suppression imaging is used before and after contrast enhancement. This technique suppresses the normal, fatty tissue of the breast, allowing easier identification of malignant masses through contrast enhancement (Fig. 10-8). MRI is also used to detect faulty or leaking breast implants. Again, fat suppression imaging is used to suppress the normal breast tissue and detect the presence of silicone in surrounding tissue. Congenital anomalies of the female reproductive system occur in approximately 1% to 2% of women in the United States. The most common anomaly is the bicornuate uterus, in which paired uterine horns extend into the fallopian tubes (Figs. 10-9 and 10-10). A unicornuate uterus occurs when the uterine cavity is elongated and has a single fallopian tube emerging from it. Often, the kidney on the side of the missing fallopian tube is also absent. Uterus didelphys is a rare congenital anomaly with complete duplication of the uterus, cervix, and vagina. The most serious complication of these anomalies is problems with reproduction, although various surgical corrections can be performed.
Reproductive System
Anatomy and Physiology
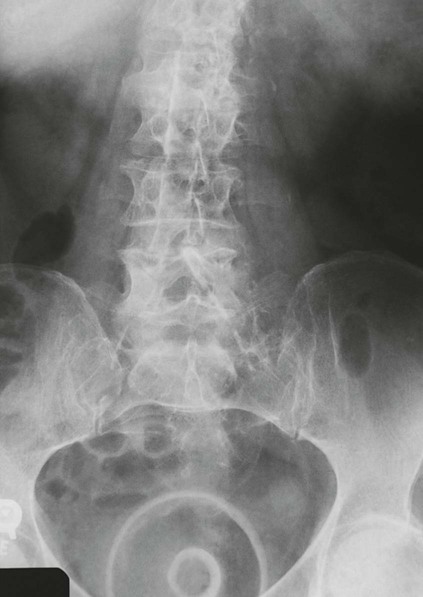
Imaging Considerations
Hysterosalpingography
Magnetic Resonance Imaging
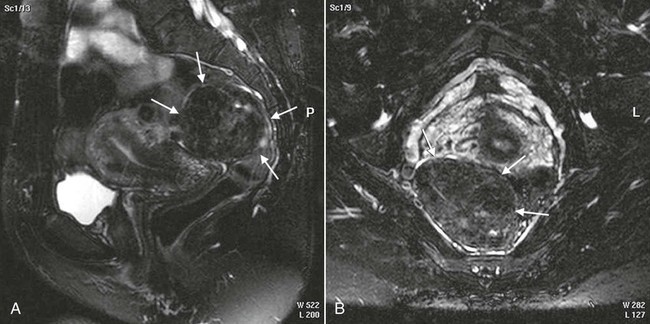
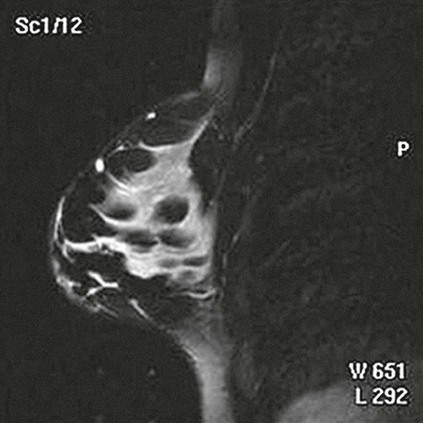
Congenital Anomalies
Reproductive System