Fig. 1
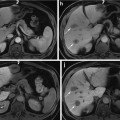
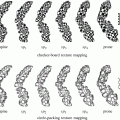
Pancreatic cancer, arising in the head of the pancreas with biliary tree dilatation and main pancreatic duct dilatation, “double duct sign.” Immediate postgadolinium T1-weighted fat-suppressed SGE images (a) fat-suppressed T2-weighted SS-ETSE, (b) coronal T2-weighted SS-ETSE (c) thick section MRCP (d). A 2-cm tumor is shown in the pancreatic head with minimal peripheral enhancement on the early post-contrast images (arrow (a)), causing proximal dilatation and abrupt distal narrowing of the common bile duct (CBD), with simultaneous dilatation of the main pancreatic duct (MPD), which represents the “double duct sign” (d)
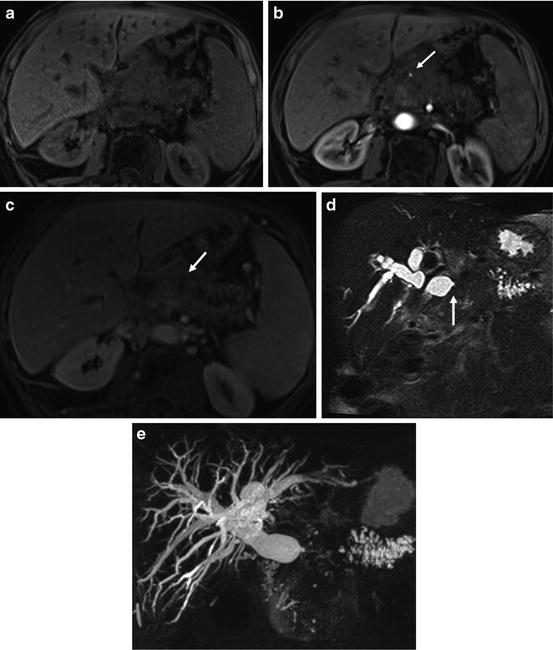
Fig. 2
Large infiltrative pancreatic cancer arising from the neck and body of the pancreas with biliary tree and pancreatic duct dilatation and vascular encasement. Fat-suppressed T1-weighted SGE (a), immediate postgadolinium T1-weighted fat-suppressed SGE (b), 90-s postgadolinium fat-suppressed SGE (c), non-breath hold 3D MRCP; and (d) MRCP with MIP reconstruction (e) images. A 5-cm poorly differentiated infiltrative adenocarcinoma of the neck and body of the pancreas (arrow (c)), causing a marked dilatation of the intrahepatic ducts and obstruction of the common bile duct (CBD) visualizing and abrupt narrowing of the distal CBD (arrow (d)). Dilatation CBD, the main pancreatic duct and side branches of the body and tail of the pancreas are best depicted on MRCP images (d, e). The tumor has low signal intensity on immediate post-contrast (b) showing encasement of the hepatic artery (arrow (b)). The portal vein and proximal SMV are encased by the mass and there is a filling defect in the SMV, compatible with thrombus (c)
Pancreatic cancer arising in the head of the pancreas may cause obstruction of the CBD and pancreatic duct, with the MR cholangiopancreatography (MRCP) appearance of a “double duct sign” (Fig. 1), which was first described on ERCP studies. This sign can be also appreciated, although less commonly, in patients with focal pancreatitis. Painless jaundice is the classical presenting feature of carcinomas within the pancreatic head. It should be noted that cancer arising in the uncinate process may not obstruct the CBD until very late in the course of disease.
Carcinoma involving the body and tail of the pancreas grows insidiously and often has already metastasized widely at the time of diagnosis [5]. The most common sites of metastases are the liver, regional lymph nodes, peritoneum, and lungs.
The rich lymphatic supply and lack of pancreatic capsule account for the early spread of cancer into regional nodes. The nodal groups most frequently involved include paraortic, parapancreatic, paracaval, celiac, and paraportal.
Clinical manifestations may be nonspecific, including abdominal pain, weight loss, and painless jaundice caused by obstruction of the common bile duct.
Nonresectable disease is seen at presentation in 75 % of patients, with liver (Fig. 3) and peritoneal metastases as the most frequent secondary organ involvement [4].
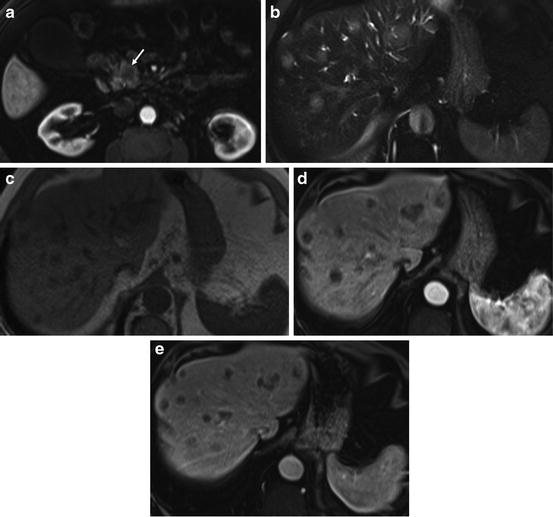

Fig. 3
Staging pancreatic cancer liver metastases. Immediate postgadolinium fat-suppressed SGE (a), T2-weighted fat-suppressed spin-echo (b), T1-weighted in-phase SGE (c), immediate, and (d) and 90-s postgadolinium T1-weighted fat-suppressed SGE images (e). Small tumor in the head of the pancreas (arrow (a)) with the presence of multiple liver metastases that are mildly hyperintense on T2 (b) hypointense on T1-weighted images (c), and have a discrete ring enhancement on the immediate postgadolinium images (d)
Surgery remains the sole curative treatment for patients with pancreatic carcinoma, with postoperative 5-year survival rate of 20 % [6].
Accurate detection and staging of pancreatic cancer are essential to ensure appropriate selection of patients who will benefit from surgery.
One study regarding prognostic factors after a Whipple procedure found that the 5-year survival rate was greater for patients with node-negative and small tumors (<3 cm) than for those with node-positive and large tumors [7]. Another study demonstrated a 5-year survival of 100 % for patients with a tumor smaller than 1 cm and limited to the intraductal epithelium [8].
Advances in magnetic resonance imaging (MRI) including fast acquisition methods and sequences, parallel imaging techniques, multichannel phased-array torso coil, and high-field MR systems (1.5 and 3.0 T) have provided high-quality images of the pancreas, which are sufficient to detect and characterize focal pancreatic lesions smaller than 1 cm. Tumors that measure <1 cm in size that are resected are associated with a 95 % 5-year survival. Hence, detection of these small tumors is critical to improve patient survival.
Magnetic Resonance Imaging Technique
New MRI techniques in the abdomen that limit artifacts have improved the role of MRI in the diagnosis and characterization of pancreatic disease.
MRI of the pancreas should always be performed with a high-field (1.5 or 3.0 T) magnetic resonance unit with a phased-array torso coil to maximize signal to noise ratio, improving breath hold and increased fat-water frequency shift, which facilitates chemically selective excitation-spoiling fat suppression or water excitation.
The use of high spatial resolution MR imaging at 3.0 T will provide the highest image quality and spatial resolution of the pancreas, allowing for the detection of small focal pancreatic lesions [9].
Breath-hold T1-weighted gradient-echo sequences obtained either as a 2D or 3D gradient echo, fat suppression techniques, and dynamic administration of gadolinium chelate provide a high-quality study of the pancreas sufficient to evaluate and characterize focal pancreatic mass lesions smaller than 1 cm in diameter and to evaluate diffuse pancreatic disease [10–12].
MRCP depicts well the biliary and pancreatic ducts to evaluate ductal dilatation (Fig. 1) and abnormal duct pathways [13–15]. Combining the tissue imaging sequences and MRCP gives comprehensive information of the full range of pancreatic disease.
The standard MR protocol for the evaluation of the pancreatic cancer used in our institution includes coronal and transverse T2-weighted single-shot echo train spin-echo (SS-ETSE), transverse T2-weighted fat-suppressed SS-ETSE, transverse T1-weighted spoiled gradient echo (SGE) in-phase and out-of-phase, and transverse T1-weighted fat-suppressed 3-dimensional gradient echo (3D GE) acquired before and after contrast administration during the hepatic arterial dominant (15–20 s), early hepatic venous (45 s), and the interstitial phase (120 s), with the interstitial phase images acquired in transverse and coronal plane.
Magnetic resonance cholangiopancreatography (MRCP) acquired in a coronal oblique projection can delineate the pancreatic and bile ducts [10] (Fig. 2).
MRCP images can be used to position in plane the pancreatic duct, in an oblique coronal projection, to delineate longer segments of the pancreatic duct in continuity.
T2-weighted echo train spin-echo sequences such as T2-weighted half-Fourier acquisition snapshot turbo spin-echo (HASTE) provide anatomic display of the common bile duct (CBD) and pancreatic duct on coronal and transverse plane images. This sequence also provides information about the complexity of pancreatic fluid collections in the presence of complications.
T2-weighted fat-suppressed images are useful in demonstrating liver metastases, islet cell, and cystic pancreatic tumors.
Performing postgadolinium gradient-echo imaging as a 3D gradient-echo technique has advantages such as: (a) thinner sections (3 vs. 5 mm for 2D-SGE) and (b) absence of mirror artifacts from the aorta, which is problematic at 2D-SGE. The thinner sections obtainable at 3 T permit high spatial resolution reconstructions in alternative planes, which can be helpful to evaluate the portal vein.
Evaluation of Pancreatic Cancer Using MRI
Tumor Detection
The normal pancreas is high in signal intensity on non-contrast T1-weighted fat-suppressed images because of the presence of aqueous protein in the acini of the pancreatic parenchyma (Fig. 4) [10]. In elderly patients the signal intensity of the pancreas can diminish and be lower than that of the liver, reflecting changes of fibrosis secondary to the aging process [12].
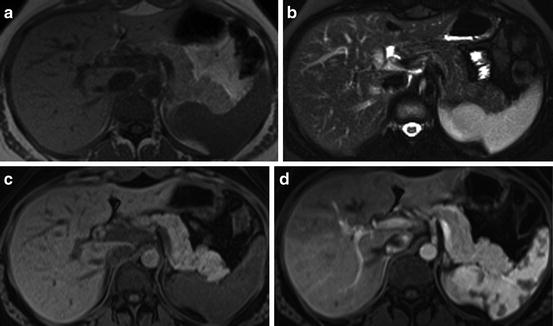

Fig. 4
A normal pancreas at 3T MRI, In-phase T1-weighted SGE (a), fat-suppressed T2-weighted SS-ETSE (b), T1-weighted fat-suppressed SGE (c), immediate postgadolinium T1-weighted fat-suppressed 3D GE images (d). Images of the pancreatic body and tail illustrates the normal appearance of the pancreas with a high intensity on T1-weighted fat-suppressed images due to the presence of aqueous protein in the acini of the pancreas (c). A uniform capillary blush is seen on the immediate postgadolinium images, which renders a higher in signal intensity comparing to the enhancement of the adjacent liver, bowel, and abdominal fat (d)
On the gadolinium contrast-enhanced images, the pancreas demonstrates a uniform capillary blush on immediate post-contrast images, becoming isointense in signal to the liver on interstitial phase images (Fig. 4) [7].
Pancreatic cancer usually appears as a low-signal-intensity mass on non-contrast T1-weighted fat-suppressed images [10, 17, 18], with decreased enhancement on immediate post-contrast images and mild progression of enhancement on interstitial phase images (Fig. 5). In the majority of cases, the tumors show margination with decreased enhancement on immediate post-contrast with a higher signal intensity of the adjacent pancreas [19]. This appearance is commonly observed in pancreatic cancer treated with chemotherapy and radiation therapy, but may also be seen at initial presentation in 27 % of the patients, which is a feature of both anaplastic and very well-differentiated tumors [19].
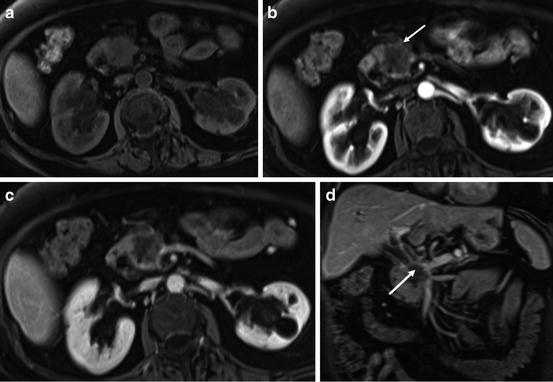

Fig. 5
Pancreatic cancer located in the pancreatic head. T1-weighted fat-suppressed SGE (a), immediate postgadolinium T1-weighted SGE (b), and transverse (c) and coronal (d) interstitial postgadolinium fat-suppressed SGE images. There is a 2-cm hypoenhancing mass in the pancreatic head clearly shown in the immediate postgadolinium image (arrow (b)) with discrete progressive enhancement on the interstitial phase gadolinium-enhanced fat-suppressed images (c). The tumor infiltrates distally the CBD, causing biliary obstruction and vascular infiltration of the main portal vein and SMV best visualized in the coronal image (arrow (d))
Conventional spin-echo images are generally limited in the detection of pancreatic cancer. On T2-weighted images, tumors are usually minimally hypointense relative to pancreas and therefore difficult to visualize.
Detection of carcinoma is best performed on immediate postgadolinium T1-weighted gradient-echo images, on which the lesion will enhance to a lesser extent than the surrounding normal tissue due to the abundant fibrous stroma and sparse tumor vascularity of the lesion [17]. The appearance of adenocarcinoma on the interstitial phase (>1 min postgadolinium) of mild progressive enhancement reflects the increased volume of the extracellular space and the venous drainage of cancers compared to normal pancreatic tissue (Fig. 5) [17].
In general, large pancreatic tumors tend to remain mildly low in signal intensity on interstitial phase images, whereas the signal intensity of smaller tumors may range from hypointense to mildly hyperintense on this phase.
Pancreatic cancers appear as mildly low-signal-intensity masses on non-contrast T1-weighted fat-suppressed images and distinct from normal pancreatic tissue, which is high in signal intensity (Fig. 5) [7, 17, 18].
Obstruction of the main pancreatic duct caused by the neoplasm, results in tumor-associated pancreatitis, which is most often observed in large tumors as the time delay during growth of the tumor allows progressive duct obstruction, atrophy, and resultant chronic pancreatitis. Pancreatic tissue distal to pancreatic cancer is often atrophic in volume and lower in signal intensity compared to normal pancreatic parenchyma due to chronic inflammation associated with progressive fibrosis and diminished proteinaceous fluid of the gland (Fig. 6) [17, 18].
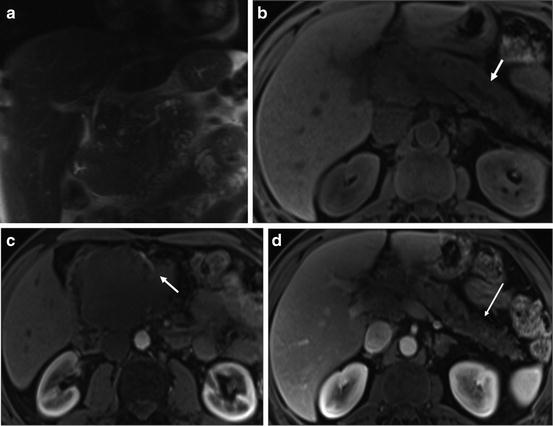

Fig. 6
Large pancreatic head cancer with tumor-associated chronic pancreatitis. Coronal T2-weighted single-shot echo train spin-echo (a), T1-weighted fat-suppressed SGE (b), immediate (c), and 90-s postgadolinium T1-weighted fat-suppressed SGE images (d). A 9-cm hypoenhancing mass located in the head and uncinate process of the pancreas encasing the superior mesenteric artery (arrow (c)) and obstruction of the distal CBD. Note the atrophy of pancreatic body and tail of the pancreas with mild dilatation of main pancreatic duct (arrow (b)). There is a diffusely low signal intensity on the precontrast T1-weighted fat-suppressed images (b), and diffuse diminished enhancement of the body and tail of the pancreas on the 90-s postgadolinium T1-weighted fat-suppressed SGE images (long arrow (d)). These findings are related to a long-term evolution pancreatic tumor-associated chronic pancreatitis
In these cases, depiction of cancer is poor on non-contrast T1-weighted fat-suppressed images. However, immediate post-contrast images can define the size and extent of adenocarcinomas that obstruct the pancreatic duct, as tumors almost always enhance less than adjacent chronically inflamed pancreas [17, 18].
Chronic pancreatitis can be seen as a focal mass-like lesion in the head of the pancreas, which appears as a low-signal-intensity mass on non-contrast and immediate post-contrast T1-weighted images. This MRI feature causes difficulty distinguishing between pancreatic cancer from chronic pancreatitis on the basis of extent of enhancement of the lesion [20, 21].
One study evaluated the accuracy of MRI, emphasizing on the T1-weighted 3D GE sequence, in the differential diagnosis between pancreatic carcinoma and chronic pancreatitis, in patients with focal pancreatic mass [22]. The study results showed a sensitivity of 93 % and specificity 75 %. The most discriminative finding for pancreatic adenocarcinoma was a relative demarcation of the mass compared to background pancreas in contrast to chronic pancreatitis. In contrast, the discriminative feature of chronic pancreatitis was an ill-defined demarcation with relatively increased signal intensity and enhancement compared with the background pancreas on early hepatic venous phase images reflecting a more progressive enhancement of inflammatory tissue compared to cancer from early to late post-contrast images. An additional helpful imaging feature is effacement of the fine, lobular architectural pattern of the pancreas in pancreatic adenocarcinoma [22], which is often preserved in pancreatitis.
The presence of encasement of the celiac axis, superior mesenteric artery (SMA), lymphadenopathy, and liver metastases are all helpful to establish the diagnosis of pancreatic cancer [23, 24].
MRI is a more reliable diagnostic technique than computed tomography (CT) in the detection of pancreatic cancer due to the superior soft tissue contrast and the multiple types of data acquired. This is especially important for small non-contour deforming pancreatic cancer, which may be difficult to identify even with multi-detector row CT [25, 26]. According to a previous study [18], the immediate post-contrast gradient-echo sequence was found to be the most sensitive approach to detect pancreatic cancer, particularly in the head region, compared to spiral CT.
Staging
Preoperative staging of pancreatic cancer is important in order to correctly select surgical candidates.
Advanced stage of disease precludes radical tumor resection in most patients with pancreatic cancer. There are several MRI findings that radiologists should address to determine resectability in the MRI report, such as: (1) distant metastases: liver, lung, and distant paraortic lymph nodes; (2) regional lymph nodes; (3) peritoneal disease; (4) direct invasion of adjacent organs: stomach, colon, spleen; (5) invasion into the peripancreatic arteries: celiac trunk, hepatic artery, SMA; and (6) invasion into peripancreatic veins: portal and superior mesenteric vein [24].
Local extension of cancer and lymphovascular involvement can be evaluated on non-suppressed T1-weighted images [26]. Low-signal-intensity tumors that extend beyond the pancreas and invade adjacent organs are detected in the background of high-signal-intensity fat tissue on nonfat-suppressed T1-weighted images [17




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree