Fig. 1
Biocontinuum of adverse early and late effects of the upper digestive and respiratory system (with permission from Rubin and Casarett 1968)
2 Anatomy and Histology
2.1 Anatomy of the Pharynx and Larynx
The pharynx is a fibromuscular tube extending from the base of the skull to the esophagus, regulating the flow of food and air in the upper aerodigestive passage (Fig. 2). The pharynx is composed of three sections: (i) the nasal, (ii) the oral, and (iii) the laryngeal pharynx.
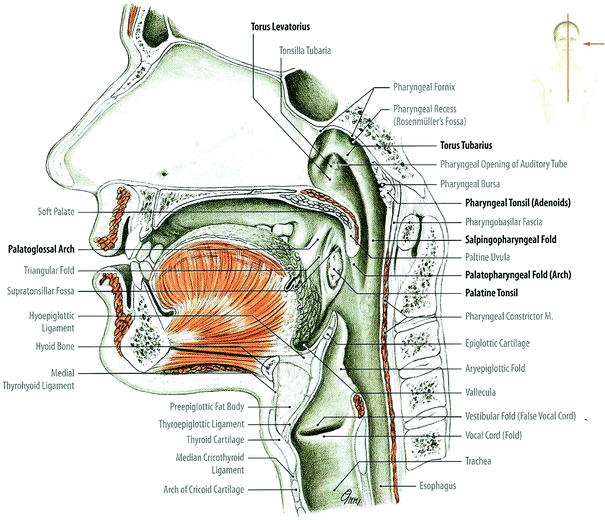

Fig. 2
Nasal and oral cavities, pharynx and larynx: median sagittal section through head and neck, medial view of the right half (with permission from Tillman 2007)
In the coronal plane from a retropharyngeal view: There are four major sphincters in the pharynx that regulate the passage of air and food to their ultimate destinations. The sphincters, at the point of the entry, are the buccopharyngeal sphincters guarding the communication between the mouth and the pharynx (fauces). They are formed by the base of the tongue and the oropharyngeal bar, which is produced by a synchronized peristaltic contraction of the posterior oropharyngeal muscular wall.
The hypopharynx is the lower continuation of the pharyngeal tube and is anatomically defined laterally and posteriorly by the middle and inferior constrictor muscles and anteriorly by the hyoid bone, thyroid, and cricoid cartilage. If the larynx were postnasal, anatomy would be less complex and the oropharynx and hypopharynx functionally and structurally would be one. The descent of the larynx transforms the tube into a series of symmetrical gutters surrounding the larynx. In the coronal plane from a retropharyngeal view, the hypopharynx is best understood anatomically from a posterior coronal view, obtained by separating the inferior constrictor muscles at the midline. The hypopharyngeal sphincters and the piriform recesses circumvent the larynx to lead food into the esophagus. The recurrent laryngeal nerve enters the larynx and can be trapped by piriform sinus cancers (Rubib and Hansen 2007).
The larynx is divided into three parts: (i) supraglottis, (ii) glottis, and (iii) subglottis; these three parts are known as the vestibule, ventricle (glottis), and infraglottic cavity. The epiglottis is readily visualized at its vestibule. The opening of the larynx, referred to as the aditus larynges or the superior laryngeal aperture, can be traced from the epiglottis to the arytenoids. The aryepiglottic folds start at the free edge of the epiglottis and terminate at the corniculate and arytenoids cartilages. The false cords and the true cords are separated by the ventricle. The true cords act as a sphincter that closes off the airway (Rubin and Hansen 2007).
2.2 Histology of the Pharynx and Larynx
Pharynx: The pharyngeal mucosa consists of nonkeratinized stratified squamous epithelium. The epiglottis contrasts the transition from the pharyngeal to laryngeal mucosa. On the oral side, the epithelium is thick with connective tissue papillae beneath it. On the laryngeal side, however, the epithelium is much thinner and has no papillae. The laryngeal side is also characterized by taste buds scattered among the epithelial cells. At the base of the epiglottis on the laryngeal side, the stratified squamous epithelium undergoes a transition into ciliated pseudostratified columnar epithelium (Fig. 3).
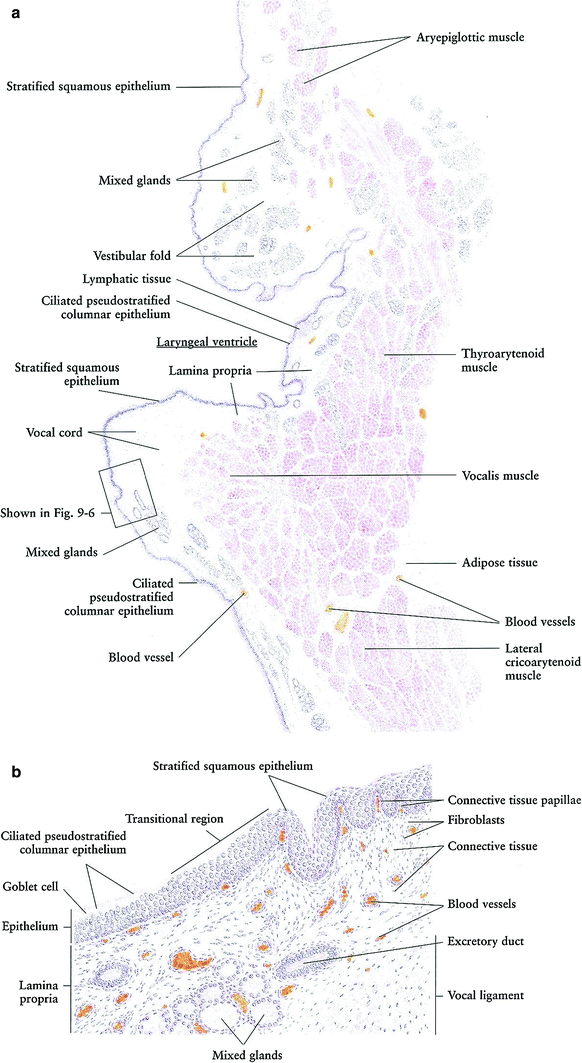

Fig. 3
a Larynx: frontal section, very low magnification. b Larynx: mucosa, high magnification (with permission from Zhang 1999)
In the lamina propria, diffused lymphatic tissue (infiltration) may be found just beneath the epithelium. The lamina propria is a loose connective tissue with blood and lymphatic vessels, adipose cells, and nerve fibers. The epiglottis glands are of mixed type, and predominantly located on the laryngeal side. The core of the epiglottis is occupied by a large area of elastic cartilage with its perichondrium bound to the deepest layer of the lamina propria.
During swallowing, the epiglottis is pressed by the base of the tongue toward the posterior wall of the pharynx, thus closing the larynx. As a result, the bolus of food is pushed to slide through the oral surface of the epiglottis and to enter the esophagus, not the trachea (Robbins et al. 1992).
Larynx: The larynx is a complex hollow organ that connects the pharynx above and the trachea below. Its lumen is covered by a mucosa lined with ciliated pseudostratified columnar epithelium and stratified squamous epithelium (Fig. 3a, b). The wall of the larynx is supported by a group of cartilages and a group of skeletal muscles.
The mucosa of the larynx forms two pairs of folds: the upper are the vestibular folds (false vocal cords) and the lower are the vocal cords. Between these two pairs of folds is the laryngeal ventricle with its narrow pouchlike prolongation, the ventricular recess. The vocal cord and vestibular fold are covered by nonkeratinized stratified squamous epithelium, while the rest is lined by ciliated pseudostratified columnar epithelium with goblet cells. The beating direction of the cilia is toward the pharynx, moving foreign particles, bacteria, and mucus toward the exterior. The lamina propria is a loose connective tissue containing blood vessels and diffused lymphatic tissue. Within the lamina propria are numerous mixed glands, except where the vocal cords are present. The core of the vocal cord is composed of vocal ligaments with bundles of elastic fibers.
Besides the vocal cord ligament is the vocalis muscle, which controls the tension on the vocal cords and is therefore associated with phonation. The other skeletal muscles surrounding the larynx include the aryepiglottic muscle, thyroarytenoid muscles, and lateral cricoarytenoid muscle. These muscles are involved in breathing and swallowing. Between the bundles of muscles is a thin layer of loose connective tissue that contains blood vessels and adipose cells. The major cartilages supporting the wall of the larynx are the thyroid, the cricoid, and the arytenoids. These are all of the hyaline type. Additionally, there are several small elastic cartilages: the corniculates, cuneiforms, and the tips of the arytenoid. No cartilages are shown in this illustration; for details, see a relevant anatomy textbook or atlas. The larynx is designed to produce sound, to close the trachea during swallowing to prevent food and saliva from passing down the airways to the lungs, and to function as a part of the respiratory system (Zhang 1999).
3 Physiology and Biology
3.1 Pharynx
Swallowing is a complex process that begins with the placement of food in the mouth and ends when the food enters the stomach. It involves voluntary and involuntary stages which are coordinated through several cranial nerves and a multitude of muscles that control the function of the oral cavity, the pharynx (skull base to the lower border of the cricoid), the larynx, hyoid bone, and esophagus (Logemann 1998; Goldsmith 2003).
Swallowing is initiated by the stimulation of receptors in the oropharyngeal area. Sensory impulses reach the brain stem through cranial nerves VII, IX, and X, while motor control is exercised through cranial nerves IX, X, and XII. The cricopharyngeal sphincter (CPS) relaxes as the bolus reaches the posterior pharyngeal wall before it reaches the CPS. Cranial nerve V contains both sensory and motor fibers and is important to chewing.
Swallowing physiology consists of three phases (Logemann 2007):
1.
Oral phase (1 s): The oral tongue and teeth reduce the food to a bolus. As the food is transported back toward the pharynx, receptors in the oropharyngeal mucosa trigger the pharyngeal phase.
2.
Pharyngeal phase (1 s): During this stage, the velopharyngeal port closes to prevent food from entering the nose. The hyoid bone and larynx begin their forward and superior ascent, the epiglottis is folded down to an inverted position, the tongue base moves toward the posterior pharyngeal wall, and pressure is generated by the top-to-bottom contraction of the pharyngeal constrictor muscles, which push the bolus of food toward the esophagus. Lastly, through laryngeal and hyoid elevation and anterior movement, the cricopharyngeus muscle relaxes, resulting in the opening of the CPS.
3.
Esophageal phase: When the CPS opens, the bolus of food enters the upper esophagus and is transported down to the stomach through peristalsis.
Patients with head and neck cancer tend to be elderly. With advanced age, swallowing physiology becomes compromised, resulting in increased bolus “holding”, delayed onset of swallow, slower pharyngeal transit time, and reduced generation of pharyngeal pressure (Tracy et al. 1989; Robbins et al. 1992).
3.2 Larynx
The Larynx is the entry to the respiratory system and serves as the organ for speech (phonation). Expired air causes the true vocal cords to vibrate; the vibrations are modulated by varying the tension in the vocal cords and changing the degree of glottis opening. It is the alteration of vibrations that produces sounds of varying pitch. The false cords have no intrinsic musculature to modulate phonation. However, they and the ventricle that separate true and false cords create sound resonance.
Vocal function endpoints include objective measurements through instrumental assessment, subjective measurement related to patient-reported items, and observer-assessed scores on communicability.
Objective evaluation: Instrumental Assessment
a.
Videostroboscopy patients are asked to produce standard vocal samples while videostroboscopy is performed. Images are obtained using a laryngoscope and a rhino-larynx stroboscope. Through this procedure, several parameters can be evaluated: supraglottic activity, vocal fold edge, amplitude, mucosal wave, phase symmetry, and glottic closure (Hirano 1981).
b.
Aerodynamic measurements (Fung et al. 2001): a pressure flow device can be used to obtain and analyze the aerodynamic productions of each patient. Patients are asked to sustain a vowel or to repeat a syllable at a predetermined rate at a comfortable intensity level. Maximum phonation time, average airflow during sustained vowel production, vocal fold diadochokinetic (VFDDK) temporal rate, and the average airflow rate on VFDDK can be measured.
c.
Acoustic analyses (Fung et al. 2001): analysis of some macro- and micro-analytical acoustic parameters such as fundamental frequency and phonational frequency range, measures of perturbation, and measures of noise.
Subjective evaluation: self–assessed questionnaire
a.
The Voice Handicap Index (VHI) (Rosen et al. 2000; Jacobson et al. 1997): it is a 30-item validated questionnaire derived for patients with benign disorders and it includes a series of questions about patient’s perceptions of their voice quality. Social-emotional and physical handicap are considered.
b.
The voice–Related Quality of Life (VR–QOL) (Hogikyan and Sethuraman 1999): it is a 10-item self-administered validated questionnaire. Social-emotional and physical functioning are included.
c.
d.
The Voice Symptom Score (VoiSS) (Funk et al. 2004) is a rigorously evaluated and psychometrically robust measure for the self-assessment of voice quality. It comprises 44 items which assess voice impairment, emotional reaction, and related physical symptoms. It differs from the other three questionnaires as it addresses other symptoms which may arise from the laryngopharynx.
Subjective evaluation: observer–assessed
a.
Communicative suitability (Franken et al. 1997; van der Torn et al. 1997): a panel of untrained volunteers judge the voice samples on communicative suitability in three different, demanding speaking situations, ranging from low demanding (talking about everyday events with a friend), medium demanding (asking a passer-by for directions), and to highly demanding (giving a lecture).
3.2.1 Definition of Laryngeal Anatomical Structures
Vocalization is a complex process that involves multiple anatomical structures which act in coordination. Due to this complexity, the definition of the most important anatomic sites whose dose-volume parameters would have a major effect on vocal function has been studied only in recent years and it is still controversial.
Dornfeld et al. (2007) considered various structures in the head and neck to be related to vocal injury: the superior and inferior base of tongue, the epiglottis, the lateral pharyngeal walls at the level of the inferior base of tongue, the pre-epiglottic space, the aryepiglottic folds, the false vocal cords, the lateral pharyngeal walls at the level of the false vocal cords, and the upper esophageal sphincter.
Sanguineti et al. (2007) evaluated the correlation between dose-volume parameters and edema, and contoured the larynx from the tip of the epiglottis superiorly to the bottom of the cricoid inferiorly; the external cartilage framework was excluded from the laryngeal volume.
3.3 Biology
Sonis has described a five-phase oral mucositis pathogenesis model that includes initiation, message generation, signaling and amplification, ulceration, and healing. Initiation occurs after administration of cytotoxic CT as a result of DNA damage and the generation of reactive oxygen species (Fig. 4). The relatively acute inflammatory or vascular phase occurs shortly after CT or RT administration. Message generation involves the upregulation of transcription factors, including nuclear factor kD(NF-kB) and activation of cytokines and stress response genes. Signaling and amplification involves the production of proinflammatory cytokines released from epithelial tissue, including TNF-α, which is related to tissue damage, and interleukin-1 (IL-1), which incites the inflammatory response and increases subepithelial vascularity that may lead to increased local CT levels (Devita et al. 2011) (Fig. 4).
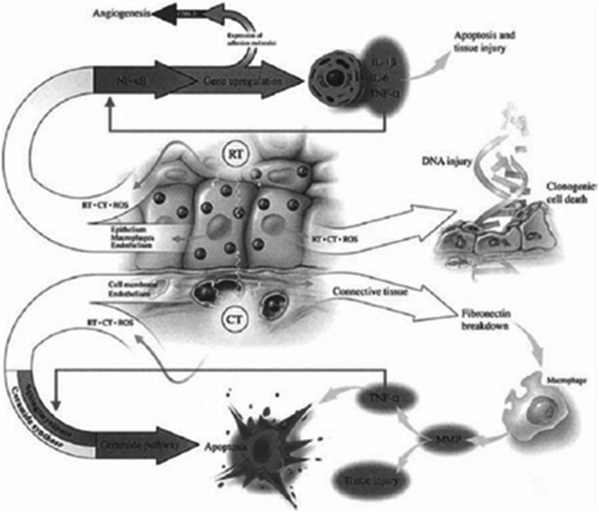

Fig. 4
Radiotherapy (RT) and chemotherapy (CT) generate ROS resulting in direct DNA injury as well as stimulation of secondary mediators leading to apoptosis. Other genes are also upregulated leading to angiogenesis. (Reprinted from Sonis et al. (2004), with permission.)
4 Pathophysiology
4.1 Pharynx
Acute: the time course for expression of pharyngeal mucositis parallels those in the oral cavity. The intensity of the mucosal reaction is dose time dependent on fractionation schedules. With more aggressive schedules and combinations of chemoradiation interferes with feeding. Most pharyngeal cancers resolve and the mucosa regenerates. However, posterior wall cancers post-cricoid in location seldom heal and can persist.
Late: treatment with nasogastric feeding tube can result in scarring and structure at the cricopharyngeus sphincter at the esophageal inlet. The origin of the esophagus is vulnerable, because the posterior wall is deficient of muscular wall in V-shaped triangle of Laimer. Recent dose/volume analysis of proximal esophagus was equivocal (Eroschenko 2007).
4.2 Larynx
Acute: the early lesions are similar to the mucositis of the pharynx and larynx with the onset varying with the intensity of the radiation schedule as documented by early investigators and continuing with the investigation of numerous fractionation schedules: i.e., hypofractionation, hyperfractionation, split course, accelerated with concomitant boost, etc. The major concern is laryngeal edema which usually subsides within 6–8 weeks, if tolerance has not been exceeded. If edema persists or anticipated, a tracheostomy is performed.
Late: the later fibrogenic phase can lead to accumulation of collagen and webbing of the vocal cord, most often at the anterior commissure due to cord proximity. Atrophy of the mucosa appears as atrophic glands resembling small islands of tumor and requires careful evaluations due to varying degrees of atypia and pseudoepitheliomatous hyperplasia. The major concern is arterial neointimal proliferation, ischemia resulting in cartilage necrosis. Chondritis and perichondritis are due to loss of chondroblasts in cartilage growth zones. When frank necrosis occurs, infection is highly likely to set in accelerating the chondronecrosis. Since laryngeal cartilage ossifies, bone sequestra occur once the mucosa is ulcerated. Arytenoid cartilages (hyaline) were most commonly involved and epiglottis (elastic) cartilages are least affected.
5 Clinical Syndromes
5.1 Pharynx
5.1.1 Swallowing Disorders Induced by Radiation Alone
Radiation-induced late toxicities, including swallowing disorders, are unevenly distributed, with some patients exhibiting more cellular radiosensitivity. Conflicting data have emerged related to target-tissue sensitivity (fibroblast vs. DNA repair capacity vs. lymphocytic chromosomal damage) and late radiation damage (Rudat et al. 1999; Borgmann et al. 2002; Geara et al. 1992). Denham et al. (2001) have categorized radiation-induced normal tissue injury as direct, indirect, and functional, in addition to resulting from genetic susceptibility. A number of tumor and radiation variables (Mittal et al. 2001; Eisbruch et al. 2002; Ang et al. 1997; Maciejewski et al. 1983; Fu et al. 1995; Taylor et al. 1992) also influence the incidence of late damage. Several investigators have documented cervical and pharyngeal fibrosis and laryngeal dysfunction resulting in swallowing disorders following standard or accelerated radiation schemes (Delaney et al. 1995; Horiot et al. 1992; Nguyen et al. 1988; Olmi et al. 1990; Marks et al. 1982; Cooper et al. 1995). Therefore, it is essential to understand the biomechanics of swallowing disorders and to know the anatomical organs that are critical for swallowing and the radiation dose-volume relationship of these organs, in order to prevent and reduce the incidence of swallowing disorders and devise effective rehabilitation techniques (Mittal et al. 2003) (Table 1).
Table 1
LENT SOMA of the Upper Respiratory and Digestive System
Larynx | ||||
|---|---|---|---|---|
Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
Subjective | ||||
Pain | Occasional and minimal | Intermittent and tolerable | Persistent and intense | Refractory and excruciating |
Voice/hoarseness | Occasional hoarseness on prolonged use | Intermittent hoarseness, voice unreliable, varies in day-to-day communication | Persistent hoarseness, incapable of normal communication | Complete loss of voice |
Breathing | Occasional difficulty | Intermittent difficulty | Labored breathing | Stridor |
Objective | ||||
Edema | Arytenoids only | Arytenoids and aryepiglottic folds | Diffuse edema of supraglottis, airway adequate | Diffuse with significant narrowing of airway, <1/2 normal |
Mucosal integrity | Patchy atrophy, telangiectasia | Complete atrophy, extensive telangiectasia | Ulcer, cartilage not exposed | Necrosis, cartilage exposed |
Respiration | Dyspnea on exertion | Labored at rest | Stridor at rest | |
Management | ||||
Pain | Occasional non-narcotic | Regular non-narcotic | Regular narcotic | Surgical intervention |
Hoarseness | Rest voice, or whisper only | No talking or whispering | Laryngectomy | |
Respiration | Humidifier, steroids | Temporary tracheostomy | Permanent tracheostomy | |
Analytic | ||||
Indirect laryngoscopy | Assessment of edema, mucosal integrity, vocal cord motion, ulcer, and necrosis | |||
Direct laryngoscopy | Assessment of edema, mucosal integrity, vocal cord motion, ulcer, and necrosis | |||
CT | Assessment of edema, necrosis, and asymmetry | |||
MRI | Assessment of edema, necrosis, and asymmetry | |||
Acute dysphasia during, and soon after, RT may result from alterations in saliva, and/or short-term laryngopharyngeal edema, resulting in acute dysphagia. This is usually limited as the salivary function and edema usually resolve within a few weeks post-RT. If these issues persist, they can lead to persistent dysphagia: e.g. long-term xerostomia can hinder swallowing function.
Radiation-induced swallowing disorders can manifest months to years later as a result of extensive fibrosis and vascular and neural damage of the pharyngeal region (Dejaeger and Goethals 1995). The biomechanics of these disorders have been studied by Lazarus (1993) using VFG in a group of patients with dysphagia 10 years following radiation. These patients demonstrated a number of oropharyngeal motility disorders, including reduced tongue-base contact with the posterior pharyngeal wall, reduced laryngeal elevation, and compromised vestibule and true vocal cord closure. These disorders resulted in pharyngeal residue, which was aspirated after swallow. Despite different tumor sites, all patients exhibited similar altered biomechanics, which most likely resulted from the large radiation doses and volumes that encompassed the orolaryngopharyngeal area during treatment. In 1995, a similar observation was made by Dejaeger and Goethals (1995) who used manofluorography in a patient 5 years following radiotherapy for a pharyngeal carcinoma. Kendall et al. (1998, 2000) used VFG to evaluate 20 patients with head and neck cancer previously treated with radiation. These patients were able to maintain nutrition and none complained of dysphagia. When compared with 60 normal subjects, all 20 patients demonstrated abnormal swallow mechanics and prolonged oropharyngeal time for all bolus sizes. The onset of aryepiglottic fold closure relative to the onset of swallow was delayed and there was a trend toward delayed hyoid elevation. In this study, there was a trend toward earlier opening of the upper esophageal sphincter relative to the arrival of the bolus, most likely as a compensatory mechanism. Patients with base of tongue cancer had worse swallow mechanics compared to patients with pharyngolaryngeal cancers.
To assess pharyngeal dysfunctions following radiation, Wu et al. (2000) used fiberoptic endoscopic examination in 31 patients with dysphagic nasopharyngeal cancer, with a mean follow-up of 8.5 years following radiation. They observed pharyngeal retention (93.5 %), post-swallow aspiration (77.4 %), atrophic changes in the tongue with or without fasciculation (54.8 %), vocal cord paralysis (29 %), velopharyngeal incompetence (58 %), delay or absence of swallow reflex (87.1 %), and poor pharyngeal constriction (80.6 %).
Jensen et al. (2007) made a similar observation using functional endoscopic evaluation of swallowing (FEES) and EORTC-QOL questionnaires in 25 patients with pharyngeal cancer treated with radiation alone and followed up for a minimum of 2.5 years (mean 5 years). Of these patients, 83 % had subjective swallowing complaints. The most frequent objective finding was reduced sensitivity in the oropharynx (94 %) and reduced range of motion at the tongue base (79 %). Pharyngeal residue appeared in 88 % of these patients, 59 % experienced laryngeal penetration, and 18 % aspirated. Penetration and aspiration were observed primarily with thin liquids. All of the six patients with aspiration were smokers. Thus, based on these studies, it is generally considered that the structures are critical for the maintenance of swallowing.
5.2 Detection and Diagnosis
5.2.1 Evaluation of the Swallowing Mechanism
1.
Objective Evaluation: Instrumental Assessment (Fig. 5)
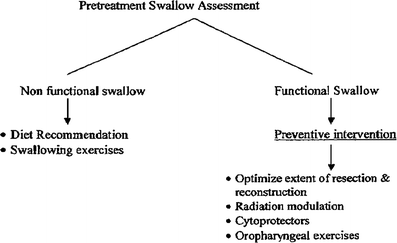

Fig. 5
Algorithm for evaluation and treatment of swallowing dysfunctions in patients with head and neck cancers
a.
Videofluorography (VFG) is the most commonly used procedure to assess swallowing dysfunctions. VFG, including modified barium swallow and esophagogram, can visualize the oral, pharyngeal, and esophageal phases of swallowing. During VFG, the patient is given food in measured volumes and viscosities. Swallowing physiology is viewed in the lateral and anteroposterior planes and temporal measures are made. The duration of physiologic events during the entire swallow can be measured as they change during swallows of boluses of various volumes and viscosities. Oropharyngeal residue and aspiration can be quantified. Oropharyngeal swallow efficiency (OPSE), a global measure of the safety and speed of swallow, is calculated by measuring the total oral and pharyngeal transit time of the bolus divided by the percentage of the bolus swallowed (Hansen et al. 2007; Zhang 1999; Devita et al. 2011; Eroschenko 2007).
b.
Manometry, in which the patient swallows a soft tube containing pressure sensors, measures pressures generated in the mouth, pharynx, and esophagus during swallowing. Manometry is used primarily to measure pressure changes in the esophagus and has value for studying oropharyngeal swallowing dysfunctions. (Ergun et al. 1993; McConnel 1988).
c.
Functional endoscopic evaluation of swallowing (FEES) provides views of the laryngopharynx different from those seen with VFG. This procedure, which is easy to perform, uses fiberoptic endoscopy (FE) to view mucosal and anatomical integrity, pharyngeal residue, swallowing with sensory testing, and aspiration (Wu et al. 2000). Wu et al. (1997) have discussed the advantages and disadvantages of using the fiberoptic endoscope versus VFG to evaluate patients with swallowing disorders.
d.
Ultrasonography can be used to study tongue physiology during swallowing (Shawker et al. 1983). However, this procedure has no value for assessing other phases of deglutition.
2.
Objective Evaluation: Observer-Assessed
Several tools are available to assess short- and long-term cancer treatment-induced swallowing dysfunctions. Common Terminology Criteria for Adverse Events (CTCAE) are frequently used to assess acute toxicity. Late toxicities can be assessed using the Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) criteria and the Subjective Objective Management Analytic (SOMA) scale (Cox et al. 1995; Pavi et al. 1995; Denis et al. 2003).
3.
Subjective Evaluation: Patient-Reported Quality of Life
Some of the instruments used to assess quality of life (QOL) in patients with head and neck cancer, including swallowing dysfunctions, include: the University of Washington Quality of Life tool (UWQOL) (Hassan and Weymuller 1993); the M.D. Anderson Dysphagia Symptom Inventory (MADSI-HN) (Rosenthal et al. 2007); the EORTC-QLQ H&N (Bjordal et al. 1995); the Performance Status Scale for Head and Neck Cancer patients (PSS-H&N) (List et al. 1996); the Radiation Therapy Instrument Head and Neck (QOL-RTI/H&N) (Trotti et al. 1998); the Functional Assessment of Cancer Therapy-H&N (FACT-H&N) (Long et al. 1996); and the Head and Neck Radiotherapy Questionnaire (HNRQ) (Browman et al. 1993). While these instruments all measure some aspects of head and neck cancer-related QOL, it is not clear which one best applies to the assessment of swallowing dysfunctions in patients with head and neck cancer and to various treatment modalities.
5.2.1.1 Baseline Swallowing Function in Patients with Head and Neck Cancer
Pauloski et al. (2000) compared 352 patients with head and neck cancer with 104 controls. Pretreatment, 59 % of patients complained of dysphagia. On VFG study, the majority of these patients had functional study suggesting inconsistency in perception of swallowing and actual swallowing ability. However, compared to controls, patients had significantly longer oral and pharyngeal transit time, greater oropharyngeal residue, and lower swallowing efficiency. Swallow function worsened with increased tumor stage and in patients with oral and pharyngeal lesions compared to those with laryngeal lesions. It is not clear if the swallowing decrement was the result of tumor infiltration of muscles and nerves or of pain and ulceration. An algorithm suggested for the evaluation and treatment of swallowing dysfunction is shown in Fig. 5.
5.2.2 Larynx
Subjective: if laryngeal cancer is present prior to treatment, it is difficult to distinguish changes due to treatment. Perhaps hoarseness and pain, which is occasional becomes intermittent and persists. Likewise, difficulty in breathing can be assessed as minimal to labored strider. Various endpoints have been suggested to assess and grade radiation effects on the larynx (Table 1a, b).
6 Radiation Tolerance
6.1 Pharynx
6.1.1 Dose Time Fractionation
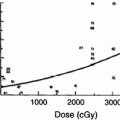
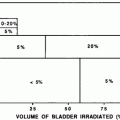
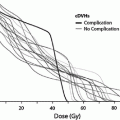
Rubin et al. (1991) suggested a 5 and 50 % risk of pharynx and larynx edema at 5 years with doses of 45 and 80 Gy, respectively. Moreover, it was felt that irradiation of less than 50 % of the larynx would not have changed these estimates (Figs. 6, 7, and 8).
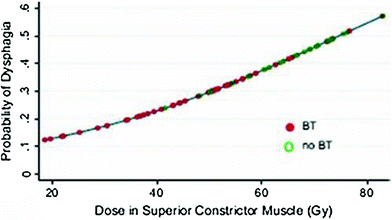
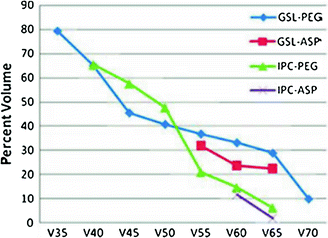
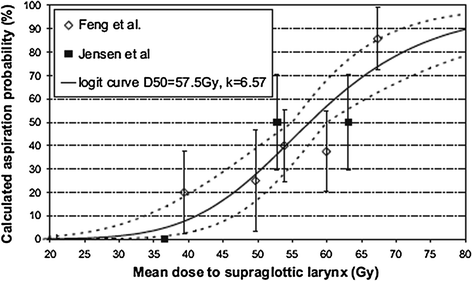

Fig. 6
Dose-effect relationship for the probability of having dysphagia (Performance Status Scale for normalcy of diet) and dose (Gy) to the superior constrictor muscle. BT, brachytherapy). (Reprinted from Teguh et al. (2008), with permission)

Fig. 7
Threshold dose-volume histogram for PEG tube dependence and aspiration (ASP). GSL, larynx; IPC, inferior pharyngeal constrictor; Vx: volume receiving x dose. (reprinted from Caudell et al. (2010), with permission)

Fig. 8
Dose-effect relationship for dysphagia according to data from Fent et al. (14) and Jensen et al. (16) Solid line fit to combined data; dotted line fit to 68 % confidence area for normal tissue complication probability-logit curve (with permission from Rancati et al. 2010)
6.1.2 Organ at Risk and the Dose-Volume-Effect Relationship
Laryngopharyngeal disorders resulting in late dysphagia and aspiration are not regimen-specific and are the result of edema and fibrosis (Pauloski et al. 1994). To correlate the relationship of radiation dose-volume-effect, it is critical to know the relative importance of the organs involved in swallowing physiology. Pauloski et al. (2006) used VFG to evaluate the “organ at risk” in 170 patients. Laryngeal elevation, tongue-base retraction, and the cricopharyngeal opening consistently predicted patients’ ability to swallow different food consistencies. Eisbruch et al. (2004) reviewed the literature and identified the structures that, if damaged, could potentially cause abnormal swallowing physiology. From their study of 26 patients assessed with VFG, direct endoscopy, and CT scan, they identified pharyngeal constrictors (PC) and glottic and supraglottic larynx (GSL) as dysphagia- and/or aspiration-related structures (DARS). In a prospective study, Feng et al. (2007) established the dose-volume-effect relationship for DARS and the esophagus in 36 patients with stage 3/4 head and neck cancers treated with chemoradiation. For intensity modulated radiation therapy (IMRT) dose optimization, planning treatment volume (PTV) was excluded from the target organ (swallowing structures, major salivary glands, and oral cavity). However, the entire organ was used to establish the dose-volume-effect relationship. A strong correlation was observed between the mean dose to DARS and dysphagia endpoints Aspiration was observed when the PC mean dose exceeded 60 Gy and the dose-volume threshold was V40 = 90 %, V50 = 80 %, V60 = 70 %, and V65 > 50 %. For aspiration to occur, the GSL dose–volume threshold was V50 > 50 % (>50 % of volume receiving 50 Gy). For stricture, a mean dose of ≥66 Gy and a dose-volume threshold of V50 = 85 %, V60 = 70 %, and V65 = 60 % for PC was observed. For stricture formation to occur, no relationship between mean dose to the GSL and esophagus was observed. The mean dose to the PC and esophagus was correlated with liquid swallowing, while only the mean dose to PC was correlated with solid swallowing on patient-reported and observer-rated swallowing scores (Table 2; Figs. 6, 7, and 8).
Table 2
Organ at risk and dose/volume relationship above which swallowing dysfunctions increases significantly (Adopted from Rancati et al. 2010, Quantec review)
Author & Number of patients | Critical organs | Dose/volume data | End point | Evaluation method | ||||
|---|---|---|---|---|---|---|---|---|
Mean dose (Gy) | Median dose (Gy) | V50 (%) | V60 (%) | V65 (%) | ||||
Eisbruch/Feng 26pts 36pts IMRT RT + Chemo | Larynx PC | >60 >66 | >50 80 85 | 70 70 | >50 60 | Aspiration Aspiration Stricture | VFG | |
Coglar 96 pts IMRT RT + Chemo | Larynx IC | <48a
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||