Key Facts
- •
Rickets and osteomalacia result from inadequate or delayed mineralization of newly synthesized organic matrix (osteoid) in mature bone (osteomalacia) or growing bone (rickets).
- •
The causes of rickets and osteomalacia fall into three main categories: (1) abnormalities of vitamin D metabolism, (2) abnormalities of calcium and phosphorus metabolism, and (3) disorders with a radiographic appearance of rickets and osteomalacia with no primary abnormalities in vitamin D or mineral metabolism.
- •
Looser zones are perpendicular areas of lucency with adjacent sclerosis that are often symmetrical, involve the pelvis, proximal femurs, scapulae, and ribs, and are typical features of osteomalacia.
- •
Hereditary causes of Vitamin D–dependent rickets are type I (pseudovitamin D–deficiency rickets) due to a deficiency in the production or activity of 1α-hydroxylase and type II (also known as hereditary vitamin D–resistant rickets).
Rickets and osteomalacia describe a spectrum of metabolic disorders with similar histopathologic and radiologic abnormalities, which result from inadequate or delayed mineralization of newly synthesized organic matrix (osteoid) in mature bone (osteomalacia) or growing bone (rickets). In other words, rickets and osteomalacia reflect the same disease process with different ages of onset.
1,25-dihydroxyvitamin D (1,25[OH] 2 D) is a steroid hormone that plays the major role in the homeostasis of calcium and phosphorus, as well as mineralization of bones. Vitamin D 2 (plant source) and vitamin D 3 (animal source) are two prohormonal forms of 1,25(OH) 2 D that should undergo hydroxylation in both the liver and kidney to become active. The major source of vitamin D in most people is supplied from conversion of 7-dehydrocholesterol in the skin to vitamin D 3 on exposure to the ultraviolet radiation in sunlight. Season, latitude, time of day, clothing, and sunscreen use are some of the factors that may affect UV exposure. Dietary sources of vitamin D such as fish oil and fortified foods are of secondary importance. Whatever the source, vitamin D as a prohormone undergoes two sequential hydroxylations to become biologically active. The first hydroxylation occurs in liver by 25-hydroxylase, a reaction that is not rigidly regulated. The resultant product, 25-hydroxyvitamin D (25-OH-D), is the major circulating and storage form of vitamin D and a reliable indicator to assess vitamin D status. 25-OH-D is hydroxylated in the kidney by 1α-hydroxylase to produce the physiologically active 1,25(OH) 2 D. This step is tightly regulated by PTH, calcium, phosphorus, and 1,25(OH) 2 D.
The main target organs of 1,25(OH) 2 D are the intestine and bone, although the kidney and parathyroid glands have also been shown to be sites of action. In the intestine, 1,25(OH) 2 D increases the absorption of calcium and phosphorus. 1,25(OH) 2 D has two actions on the bone that may seem diametrically opposed. In the presence of PTH, it stimulates osteoclastic osteolysis, which results in release and mobilization of calcium and phosphorus from previously formed bones. 1,25(OH) 2 D also promotes mineralization of organic matrix by maintenance of adequate serum calcium and phosphorus or by exerting a direct effect on the bone (or both). This dual action plays a major role in the homeostasis of calcium and phosphorus by the skeleton. 1,25(OH) 2 D also increases the resorption of phosphate in the renal tubules and suppresses the secretion of PTH from the parathyroid glands by a negative feedback loop.
The causes of rickets and osteomalacia fall into three main categories: (1) abnormalities of vitamin D metabolism, (2) abnormalities of calcium and phosphorus metabolism, and (3) a group of disorders with a radiographic appearance of rickets and osteomalacia with no primary abnormalities in vitamin D or mineral metabolism. The etiologic factors may be acquired or inherited. Any factor that interferes with the steps in the sequence of vitamin D metabolism (intake, UV conversion of prohormone in the skin, hydroxylation in liver or kidney, and end-organ resistance due to receptor defects) can result in osteomalacia and rickets. Due to fortification of many foods with vitamin D, the incidence of inadequate intake of this vitamin is lower in the United States than in other countries. Vitamin D deficiency is mostly the combined consequence of low dietary intake (or malabsorption) and decreased biosynthesis of vitamin D in the skin following deficient sun exposure. Disorders of the pancreas and hepatobiliary system, as well as small-intestine malabsorptive states such as celiac disease, regional enteritis, and bypass surgery can result in vitamin D malabsorption and deficiency. Abnormalities in the hydroxylation of provitamin D3 in liver and kidney can also lead to osteomalacia and rickets. While dysfunction of 25-hydroxylase following severe liver disease or isoniazide treatment is an infrequent cause of vitamin D deficiency, impaired 1α-hydroxylase is common in patients with advanced renal disease. The resultant hypocalcemia following low vitamin D and phosphate retention in chronic renal failure results in secondary hyperparathyroidism, which increases calcium and phosphate release from the skeleton leading to osteomalacia and osteopenia. As phosphate retention advances, the calcium phosphate product (Ca × P) increases, resulting in calcification of soft tissues that can be detected radiographically.
OSTEOARTICULAR IMAGING FEATURES
Radiographs
Having a common pathophysiologic basis resulting in histologic and gross morphologic changes, rickets and osteomalacia have similar radiologic manifestations. However, there are some characteristic radiographic changes that can help in the diagnosis of select diseases.
Rickets
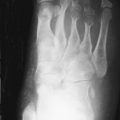
The clinical and radiographic manifestations of rickets depend on the age at which it occurs and the severity of the vitamin D deficiency; most severe changes occur if they coincide with a period of rapid growth. Retardation in body growth and generalized osteopenia owing to decreased mineralization of bones are seen. Chaotic proliferation of cartilaginous cells in the zone of maturation of the epiphyseal plate results in a bulky mass of cells in both the longitudinal and latitudinal axes in the poorly mineralized metaphysis.
The earliest radiographic finding of rickets consists of slight axial widening at the growth plate followed by a decrease in the density of the metaphyseal side of the growth plate (zone of provisional calcification). With progression of the disease, the widening of the growth plate increases and the zone of provisional calcification becomes irregular. Fraying and disorganization of the spongy bone in the metaphysis occurs. As the bulk of cell mass increases, it protrudes into the nonmineralized and weakened metaphyseal end, resulting in widening and cupping of the metaphyses. These are the characteristic radiologic findings of rickets ( Figure 35-1 ) ( Box 35-1 ). The radiographic changes of rickets are best seen in the regions with the most active growth. In decreasing order, the regions with the highest rate of growth and radiographic yield are costochondral junctions of the middle ribs, the distal femur, the proximal portion of the humerus, both ends of the tibia, and the distal ends of the ulna and radius. Because of the faster growth of distal ulna, radiographic evaluation of the ulna is more sensitive for detecting rickets than is evaluation of the distal radius. However, low production of osteoid following severe malnutrition can mask the radiographic signs of rickets.

Widening of the growth plates
Irregularity or loss of the sclerotic zone of provisional calcification
Widening and cupping of the metaphysis
Skull deformities (infancy)
Bowing deformities
Greenstick fractures
Rachitic rosary
Pectus carinatum
Scoliosis, ballooned disks
Triradiate pelvis
Slipped capital femoral epiphysis
Not all regions of the skeleton are affected equally in rickets. The radiographic changes are best seen in the regions with the most active growth such as the knees, proximal humeri, and wrists.
It has been shown that the head deformity of rickets occurs in the first months of life. The incessant overproduction and accumulation of osteoid in the frontal and parietal regions results in the prominence of frontal bones (frontal bossing), and flattening of the posterior skull, basilar invagination, and squared appearance of the skull (known as craniotabes or caput quadratum). During infancy and childhood, the long bones develop deformities both at the shaft-cartilage junction and in the diaphyses (bowing), with the weight-bearing limbs being most severely affected. While forearm deformities develop in crawling children, genu varum (“bow legs”) or genu valgum (“knock knees”) is seen in toddlers. Anterior bowing of the tibia (saber shin deformity) may also occur. Greenstick fractures may occur in the weakened long bones with no clinical symptoms, and the callus may be observed on radiographic images. There may be a delay in the eruption of the teeth with defects and hypoplasia of enamel resulting in caries. The deposition of newly formed uncalcified osteoid at the junction of the bone shafts and cartilage can lead to swelling about the joints and bead-like rounded knobs at the costochondral junctions of the middle ribs (rachitic rosary). A semicoronal impression (Harrison’s groove) may be seen over the abdomen at the level of the insertion of the diaphragm, resulting from pulling on the weakened ribs by the diaphragm during inspiration. The sternum may appear prominent and project anteriorly (pigeon breast deformity or pectus carinatum).
As age increases, weight bearing results in the development of scoliosis and, coupled with bending of the long bones, leads to a decrease in height. The intervertebral disk spaces expand, and concave impressions are formed on endplate surfaces of vertebral bodies, giving them a biconcave appearance. A promontory can be seen following the intrusion of the spine into the soft pelvis, producing a triradiate configuration. The anteroposterior diameter of the pelvis may diminish as a result of scoliosis. Slipped capital femoral epiphyses may also be noted on radiographic images.
It takes several months for the osseous changes of vitamin D–deficiency rickets to be apparent radiographically. Although rickets may arise within 2 months in breast-fed infants of vitamin D–deficient mothers, it usually develops toward the end of the first year and during the second year of life.
Rickets usually develops toward the end of the first year and during the second year of life.
Osteomalacia
Osteopenia, a decrease in the radiographic bone density, is a general and nonspecific finding in osteomalacia. A coarsened and indistinct trabecular pattern with “fuzzy” or unsharp margins is seen, which can be useful in differentiation of osteomalacia from osteoporosis. Skull, spine, long bones, and pelvis may develop the deformities discussed in rickets ( Box 35-2 ).
Decreased bone density
Blurring of trabeculae
Bone deformity (e.g., bowing)
Looser zones
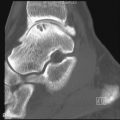
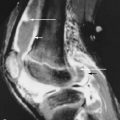
Looser zones or pseudofractures, the classic radiographic findings of osteomalacia are radiolucent bands perpendicular to the cortex that incompletely span the diameter of the bone. Mild to moderate sclerosis may be seen at the margins of these pseudofractures. Looser zones result from the focal deposition of uncalcified osteoid and may precede other radiographic changes. They are seen in characteristic sites such as the femoral neck ( Figures 35-2 and 35-3 ) below the lesser trochanter (subtrochantric region), the superior and inferior pubic rami, and the axillary margins of the scapula, ribs, and posterior margins of the proximal ulnae. Complete fractures may occur in the weakened areas. Pseudofractures typically appear bilateral and symmetric, which is another characteristic sign of osteomalacia and may be easily missed at early stages. Similar radiolucent areas may also be observed in Paget’s disease, fibrous dysplasia, and rarely osteoporosis. In contrast to the pseudofractures of osteomalacia, the fractures in Paget’s disease and fibrous dysplasia are confined to the affected bone and do not have a generalized pattern. At the early stages, Looser zones may be easily missed, and a bone scan can show regions of cortical abnormality that ultimately develop into Looser zones.