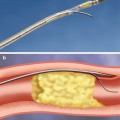
Fig. 12.1
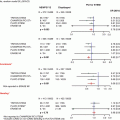
(a) Simultaneous projections of live and cine images. (b) Virtual landmark tracked on a recorded angiographic loop
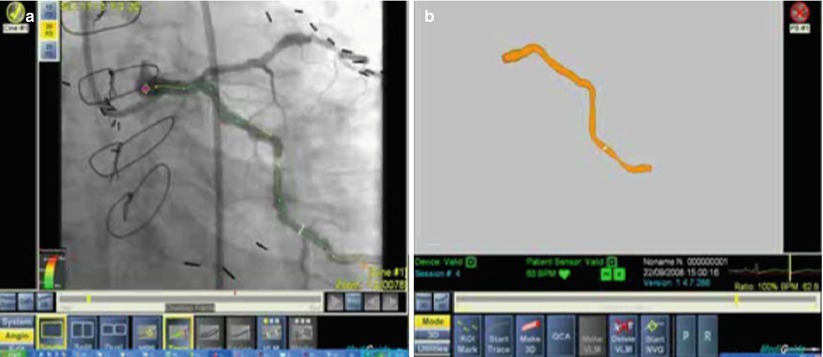
Fig. 12.2
(a) Smart trace shown on a recorded cine angiography loop. (b) In 3D Image reconstruction, a single cine-loop is used for automatic segmentation of lumen cross-section data
The system also allows the user to place one or more landmarks at points of interest while superimposing them on the angiogram (real-time and cine-loops) and on the 3D model. This mark is intended to point to an anatomical landmark and is displayed on any image and from any viewing angle. This can help the operator to position devices in accurate positions like bifurcations, aorto-ostial, overlapping stents, etc. (Fig. 12.2).
Clinical Experience
The initial preclinical experience demonstrated the safety and clinical potential of the MPS and GMC in pigs [9]. In the first human experience, 20 patients were studied in a single-center study to evaluate the safety and feasibility of the MPS system and GMC catheter. The device met all its expected primary performance end points without any related adverse events in any of the patients [10]. The system performance was evaluated on a semiquantitative one-to-five scale. The mean scores (out of maximal 5) for tracking as assessed by projection on live fluoroscopy was 4.89 and for tracking as assessed by projection on recorded cine-loop was 3.58. Length measurement of a given 20 mm distance was significantly better with the MPS (mean deviation of 0.6 mm = 3 %) as compared to standard QCA (1.5 mm = 8 %, p < 0.05). Creating a 3D reconstruction was possible in 13 out of 20 cases with an average score of 4.68.
In a more advanced 2-site study, an additional 40 patients were studied in a safety and feasibility regulatory study evaluating a newer version of the system [11, 12]. There were no complications or adverse events associated with the use of the system. Procedure success (defined as GMC performance success and freedom of MACE at 48 h) was achieved in 90 % of the patients. The GMC catheter was able to reach the distal vessel and perform the necessary measurements (i.e., assessment of minimal luminal diameter (MLD) in patients that underwent PCI) in 97.5 and 95 % of the time, respectively. The MPS accuracy was highest when the target was superimposed on the live fluoroscopy (score of 4.9 out of max 5) and the 3D vessel reconstruction (score 4.7). MLD measurements were not significantly different between the MPS system and conventional QCA. The results confirmed the functional and operational performance of the MPS-enabled GMC while demonstrating an excellent safety profile. Based on this study, the system was cleared by the FDA.
The results of these initial experience studies suggest that the use of this navigation and positioning technology can contribute to PCI procedures. For example, utilizing 3D reconstructions of the coronary tree and lesion minimizes angiographic views thereby potentially reducing radiation exposure and contrast administration. Furthermore, virtual landmarks and interventional devices with sensors can provide the operator with improved accuracy in positioning the interventional device without the need for fluoroscopy or repeat contrast injections.
The inventors of the system suggested combining it with intravascular ultrasound (IVUS) into a system called GIVUS (Guided IVUS). This system may have the potential to collect ultrasound images along their corresponding spatial orientation. GIVUS will use the near real-time segmentation algorithm to automatically identify the vessel’s lumen and external elastic membrane on the IVUS images to generate an accurate 3D model of the vessel. The initial GIVUS system was tested in animal models.
In summary, the MediGuide navigation system provides a novel concept of navigation and positioning of intravascular devices. With improved precision, the system has the potential to result in significant reduction in contrast-media volume and radiation exposure. One of its major limitations is the high cost of the system, special installation and occupying space in the cath lab. However, with further development and integration with intravascular devices, the system has the potential to become a prominent tool in treating complex coronary lesions in a robotic-oriented cath lab.
Stereotaxis Magnetic Navigation System
The Stereotaxis Magnetic Navigation System (MNS) introduced a new concept of manipulating devices inside the heart and blood vessels. This technology uses a magnetic field around the patient, affecting the metal devices inside the body. The operator can change the orientation of the magnetic field, which then changes the position and orientation of the device. By changing the magnetic field, the operator specifically orients the catheter. The MNS has gained wide popularity mainly in the electrophysiology laboratory, combining an ablation catheter with this system, and has been also introduced to the coronary and vascular fields.
Magnetic Navigation System
The MNS is an integrated system consisting of a workstation and magnet control equipment that navigates catheters and guidewires through small magnets placed at their distal tips. These small magnets are then precisely oriented by manipulating the magnetic field generated by two large permanent magnets mounted on mechanical positioners on either side of the fluoroscopy table. Rotation and translation of these magnets maintain a magnetic field of 0.08 T in strength and have a radius of 15 cm over the patient’s heart. The magnets can move in three different coordinates, rotate, move toward and from the navigation volume, and tilt. This allows the magnetic field vector to be oriented in full 360°. The two table-side magnets can be rotated into position or parked away when not in use.
The operator uses a navigation workstation that utilizes a software interface that co-registers the coronary imaging with the 3D navigation of the magnetic system. The imaging includes the coronary angiogram as well as a 3D registration of a coronary computerized tomographic scan and the Paieon imaging reconstruction software. These build the 3D vessel road mapping for the magnetic-assisted interventions (Fig. 12.3). With the 3D vessel reconstruction on the screen, the operator can form and direct the device magnetic vector path along the long axis of the coronary arterial vessel (Fig. 12.4) and direct the wire tip longitudinally. The system allows angles of up to 120° from the long axis, facilitating access to very complex coronary angulations.
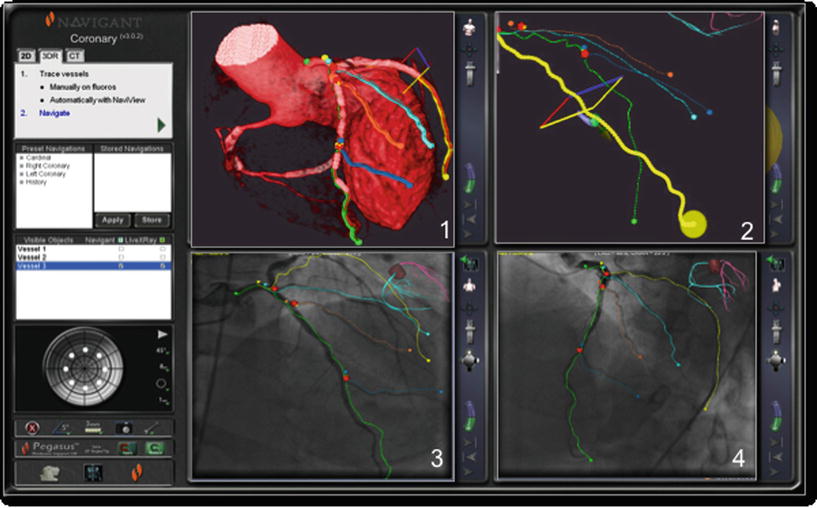
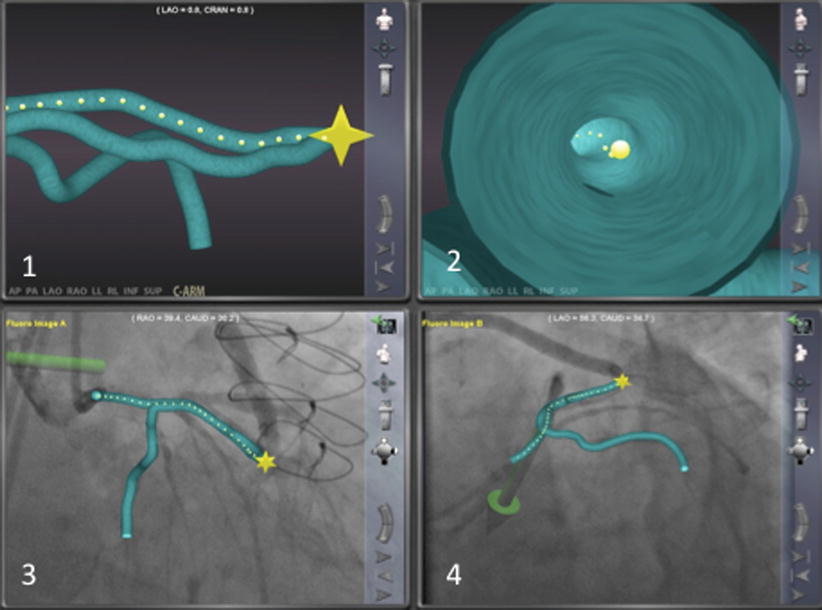

Fig. 12.3
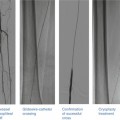
User interface example for left coronary artery. The display shows various options: Three-dimensional navigation using multislice computer tomography (1, 2) and two-dimensional angiography-based navigation (3, 4)

Fig. 12.4
This shows the three-dimensional reconstruction created from the angiogram using the Navigant software system. The system also provides an endoluminal view as well as the opportunity to superimpose the three-dimensional images on the angiographic images
Magnetic Wires
The magnetic tipped guidewires of the Pegasus family are made in a wide range of shaft support and tip stiffness for different interventional needs. They are blended with nitinol and stainless steel, with the distal 2 mm serving as the magnetic tip. As described above, these wires are precisely oriented through vessels by the MNS. The operator manually advances the wire but utilizes the software to help guide the direction of the wire. This is particularly important in complex anatomies with severe angulations.
Clinical Experience
The use of the MNS has been shown to be quite effective in several fields other than interventional cardiology. For example, in electrophysiology, the MNS has been successful for mapping and ablating arrhythmias [13] even within complex atrial anatomy [14] with no associated risk of perforation [13, 15].
The initial experience in humans demonstrated the feasibility of the MNS in performing PCI including successful crossing of complex lesions where conventional wires had failed [16, 17]. Additional single-center studies further substantiated the success of the MNS in routine [18, 19] and complex lesions [17, 20–22]. In two large series, which combined over 500 patients undergoing MNS-assisted PCI, procedural success rates were 89 and 93.4 % [17, 23]. These success rates were similar to procedural success rates in standard manual PCI. The MNS system has been reported to be successful in complex coronary lesions like bifurcations [20, 21, 24], bypass grafts with loops [25], and alcohol septal ablation [26]. The big challenge remains, as in standard manual PCI, with chronic total occlusion (CTO). Case reports highlighted the potential of this technology in conquering total occlusions [27]. The use of multislice CT co-registration has importance in planning CTO procedures with the additional benefit of magnetic navigation.
The magnetic navigation system was suggested to reduce fluoroscopy time [20, 23, 25]. By reducing fluoroscopy time, it reduces radiation exposure to both the operator and patient. With further development of remote manipulation of the devices, the radiation exposure will be further reduced, and by controlling the procedure remotely from the control room, the comfort of the operator is improved. Another potential benefit of the MNS is reduction in contrast usage [20, 25], which lowers the risk for contrast-induced nephropathy.
The magnetic navigation system has been also suggested for peripheral arterial procedures. Combined with a radio-frequency ablation catheter, this system has the potential to be successful for long total occlusions with increased success and reduced burden of radiation and orthopedic stress on the operator.
In summary, the Stereotaxis navigation system provides a new avenue for treating challenging and tortuous anatomy, CTO’s, and a reduction in contrast, radiation exposure, time, and use of other resources. One of its major limitations is the high cost of the system, occupying a large space in the cath lab, and the inability to be used in patients with metal in their bodies. However, with further development of high-performance dedicated devices, advances in the 3D co-registration, progress in remote manipulation, and development of magnetic resistant pacemakers and ICDs, this system has the potential to become a prominent tool in treating complex coronary lesions in a robotic-oriented cath lab.
CorPath Robotic System
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree