Fig. 3.1
Scheme demonstrates conventional neoadjuvant chemoradiotherapy regimen. Neoadjuvant chemoradiation regimen including cycles of bolus 5-fluorouracil (5FU) and leucovorin administered every 21 days including during the “resting” period after radiation completion. RT Radiotherapy
In short-course radiotherapy, 5 Gy external beam radiation is administered daily over 5 days without chemotherapy, followed by surgery within 1 week. This regimen is mainly used in Northern Europe and Scandinavia countries. According to the Swedish Rectal Cancer Trial, compared with surgery alone, patients who received “short-course” radiotherapy had reduced local recurrence (11 % vs 27 %, p < 0.00) and improved 5 year overall survival (58 % vs 48 %, p = 0.004) [17]. The benefits of the short-course radiotherapy regimen continued at 13 years’ follow-up (local recurrence: 9 % vs 26 %, p < 0.001; overall survival: 38 % vs 30 %, p = 0.008) [18]. Patients who received Short-Course Radiotherapy , however, had a higher rate of hospitalization over the 6-month period after surgery and more gastrointestinal complications [19]. Accordingly, in the Dutch TME Trial, patients who received “short-course” neoadjuvant therapy combined with total mesorectal excision (TME) had significantly lower local recurrence compared to those who had TME alone (2.4 % vs 8.2 %, p < 0.001); however, no long-term survival benefit was found in the “short-course” group [20, 21].
The efficacy of preoperative versus postoperative chemoradiotherapy has been investigated by the German Rectal Cancer Study Group [22]. This trial randomly assigned 823 patients with T3 or T4 and/or node-positive rectal cancers to receive either pre- or postoperative chemoradiation. Chemoradiotherapy consisted of 50.4 Gy of external beam radiation in 28 fractions with concurrent 5-FU (1000 mg/m2 per day for 5 days in the first and fifth week of radiation). Total mesorectal excision was performed in all patients and all patients received additional 4 cycles of 5-FU-based chemotherapy. Local recurrence rates were significantly higher in the postoperative chemoradiotherapy group (15 %) compared to the preoperative group (6 %) (p = 0.006). Rates of disease-free survival, overall survival, and sphincter preservation did not differ between the two groups. These authors recommended that preoperative long-term chemoradiotherapy as the standard treatment for patients with locally advanced disease that requires downstaging.
Recently, a meta-analysis was performed to assess the effectiveness and safety of neoadjuvant radiotherapy in the management of rectal cancer [23]. The authors identified 17 trials comparing neoadjuvant therapy versus surgery alone. A total of 8568 patients were enrolled. In the five trials comparing neoadjuvant chemoradiotherapy to neoadjuvant radiotherapy, 2393 patients were enrolled. The investigators found that neoadjuvant radiotherapy resulted in improved local disease control (hazard ratio 0.59; 95 % confidence interval 0.48–0.72) compared to surgery alone even after total mesorectal excision, whereas its benefit in overall survival failed to reach statistical significance (0.93; 0.85–1.00). Short-course radiotherapy, however, was followed by significantly increased perioperative mortality (1.48; 1.08–2.03), particularly if a dose of 5 Gy per fraction was administered (1.85; 1.23–2.87). Chemoradiotherapy improved local control compared to radiotherapy alone (0.53; 0.39–0.72), with no impact on perioperative outcome and long-term survival. The authors concluded that neoadjuvant radiotherapy had a favorable impact on local control in patients with rectal cancer, particularly when combined chemotherapy is administered. The question of whether use of more active, modern chemotherapy protocols or targeted therapy in the neoadjuvant setting will improve overall survival after curative resection requires further investigation.
Most studies of the adverse impact of neoadjuvant therapy on perioperative morbidity and mortality occurred in patients treated before 1980. Some authors have associated the incidence of late bowel obstruction and increased postoperative mortality with the large irradiation volume, including paraortic lymph node regions [19, 24]. Radiotherapy techniques have evolved enormously since that time, including increased accuracy of target definition and more precise dose delivery, using intensity-modulated techniques. Use of these modern radiotherapy strategies need to be evaluated in future studies.
Benefits of Neoadjuvant Radiotherapy
At present, neoadjuvant chemoradiation is the preferred treatment for patients with locally advanced rectal cancer. In addition to the benefits in improving local disease control, the majority of patients receiving “long-course” neoadjuvant chemoradiation obtain significant tumor regression and downshift of their T and/or N status, leading to a downstage of the primary tumor [25]. Downsizing may alter surgical planning, particularly in low rectal tumors, by making a sphincter saving operation possible. In addition, up to 20–30 % of patients will have a complete pathological response, with no viable tumor cell found in the resected rectum [26]. Although radical surgery including total mesorectal excision remains the primary treatment after neoadjuvant chemotherapy, new local excision alternatives have been developed for selected patients.
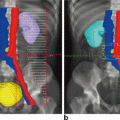
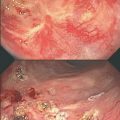
Colonoscopic and radiological aspects of incomplete clinical response after neoadjuvant chemoradiation are shown in Figs. 3.2 and 3.3. Figure 3.4 shows magnetic resonance imaging (MRI) before and after neoadjuvant chemoradiotherapy in a case of complete clinical response.
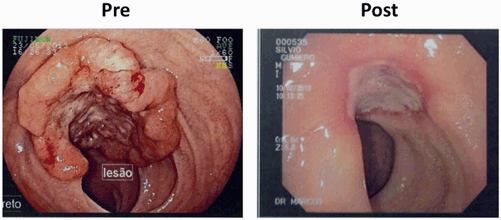
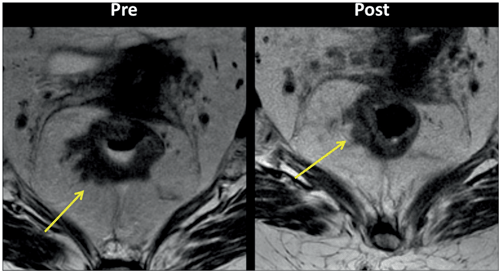
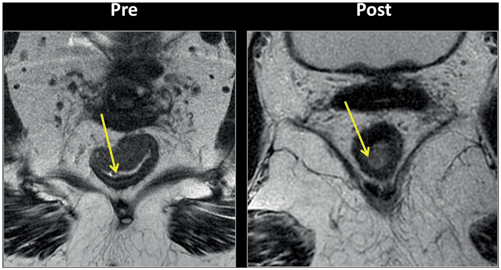

Fig. 3.2
Colonoscopic findings demonstrate incomplete response after neoadjuvant chemoradiotherapy

Fig. 3.3
Pelvic magnetic resonance imaging (MRI) findings demonstrate incomplete response after neoadjuvant chemoradiotherapy

Fig. 3.4
Pelvic magnetic resonance imaging (MRI) findings demonstrate complete response after neoadjuvant chemoradiotherapy
While long-course neoadjuvant chemoradiotherapy and short-course radiotherapy are both efficacious, there are limited data specifically comparing these two regimens. In 2006, the Polish Colorectal Study Group published the long-term results of a trial comparing short- and long-course neoadjuvant therapy in 312 patients with T3/4 mid-to-low rectal cancer [27, 28]. All patients underwent total mesorectal excision. The rates of sphincter preservation were similar in both groups [58–61 %, respectively, for the long- and short-course neoadjuvant therapy arms (p = 0.570)]. However, the circumferential margin at the time of surgery was positive in 4 % of patients receiving long-course neoadjuvant chemoradiotherapy, compared with 13 % of patients in the short-course radiotherapy group. No differences were found in local recurrence, late toxicity, or overall survival. However, complete pathological response was higher in patients receiving long-course neoadjuvant chemoradiotherapy (16.1–0.7 %, respectively). This can be explained by the fact that in the short-course protocol surgery is performed within a week after the end of radiotherapy, an approach that does not allow for preoperative downstaging.
Significant rectal tumor downstaging following neoadjuvant chemoradiation has raised the issue of offering patients with small residual cancers restricted to the bowel wall , an alternative to total mesorectal excision in order to avoid its associated morbidities, particularly urinary and sexual disturbances due to autonomic denervation. In patients who develop significant tumor regression, excision and node sterilization may be appropriate for less aggressive techniques such as transanal endoscopic microsurgery [29].
Although still considered controversial, nonoperative management of patients with a complete clinical response after completion of neoadjuvant chemoradiation is steadily increasing. In order to avoid postoperative morbidity and functional disorders, our group has considered withholding immediate surgery (the “Watch and Wait” approach) for patients with complete clinical response after neoadjuvant chemoradiotherapy for distal rectal cancer. Figure 3.5 demonstrates findings on colonoscopy, MRI, and anal ultrasound in a patient who received neoadjuvant chemoradiation.
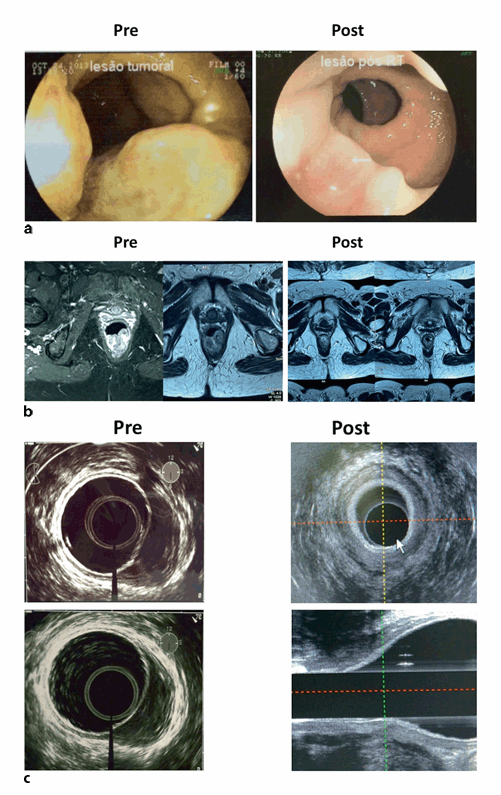

Fig. 3.5
Complete response after neoadjuvant chemoradiation: results in the same patient of colonoscopy (a), pelvic magnetic resonance imaging (MRI) (b), and endorectal ultrasound (c), before and after (10 weeks) the end of radiotherapy
In 1997, our group published the results of a study involving 118 patients with potentially resectable cases of histologically proven low rectal adenocarcinoma [25]. Treatment consisted of external beam radiotherapy 5040 cGy for 6 weeks and concurrent leucovorin (20/mg/m2/day) with bolus doses of 5-FU administered intravenously at 425 mg/m2/day for three consecutive days on the first and last 3 days of radiation therapy. After 2 months, all patients underwent repeat evaluation and biopsy of any suspected residual lesions or scar tissue [30]. Thirty-six (30.5 %) patients were classified as being complete responders. In six of these patients, complete response was confirmed by the absence of tumor in the surgical specimens (three abdominal resections and three proctosigmoidectomies with coloanal anastomosis). In the remaining 30 patients, complete response was confirmed by the absence of symptoms, negative findings on physical examination, biopsy, ERUS, and pelvic computed tomography (CT) results, during a median follow-up of 36 months. Eighty-two patients (69.5 %) were considered incomplete responders. Residual lesion was identified during the first examination in 74 patients, and after 3–14 months in the remaining 8 patients. Except for one patient who refused surgery, all patients in this group underwent surgical treatment, including coloanal anastomosis (36 patients), local excision (9 patients), and abdominal resection (4 patients). In this study, combined up-front chemoradiotherapy was associated with tolerable and acceptable side effects. Thus, our study showed that 30 patients (25 %) were spared from surgical treatment. Sphincter-saving management occurred in 38 % of patients who might otherwise have required an abdominoperineal resection. The preliminary results of this trial suggested a reduction in the number of local recurrences and reinforced the concept that in some selected cases, infiltrative low rectal cancer should be initially treated by chemoradiotherapy.
In a subsequent study, the impact of an extended neoadjuvant chemoradiation regimen on complete response rates was evaluated [31]. Radiotherapy consisted of 45 Gy, delivered by a three-field approach with daily doses of 1.8 Gy on weekdays to the pelvis, followed by a 9 Gy boost to the primary tumor and perirectal tissue (a total of 54 Gy administered over a 6-week period). Concomitantly, patients received three cycles of bolus 5-FU (450 mg/m2) and a fixed dose of 50 mg of leucovorin for three consecutive days every 3 weeks. After completion of radiation therapy, patients received three additional identical cycles of chemotherapy every 3 weeks until 9 weeks after completion of radiation therapy (Fig. 3.1). Patients with biopsy-proven resectable adenocarcinoma located no more than 7 cm from the anal verge and no evidence of systemic metastatic disease were eligible for inclusion in the study. All patients underwent complete physical examination, digital rectal examination, and rigid proctoscopy. Disease staging modalities included either pelvic MRI or ERUS with three-dimensional (3D) technology for T and N staging, in all patients. In addition, carcinoembryonic antigen (CEA) and abdominal and chest CT scans to exclude metastatic disease were performed at initial staging. Patients with radiological evidence of T3 or T4 tumors were included in this novel neoadjuvant chemoradiation strategy. Tumor response was assessed immediately after completion of chemotherapy, at 10 weeks from completion of radiation, using complete physical examination, digital rectal examination, and rigid proctoscopy. The same imaging studies that were performed at initial assessment were performed again at tumor response assessment. Patients with complete clinical response were strictly monitored and surgery was deferred to a later time. Patients with incomplete clinical response were immediately referred to surgery.
Twenty-nine patients completed 12 months of follow-up and were included in this preliminary analysis; 28 (97 %) of them successfully completed treatment. Median follow-up was 23 months. Fourteen patients (48 %) were considered as complete clinical responders sustained for at least 12 months (median, 24 months). Fifteen of 16 patients had skin-related grade III toxicities (93 %). This preliminary series suggested that extended 5-FU-based neoadjuvant chemotherapy regimens during the “resting period” after radiation completion are well tolerated and may increase radiosensitivity of the primary tumor [31].
As these studies suggest, increased rates of complete clinical tumor response are expected for Stage I through Stage III distal rectal cancers with extended 5-FU-based neoadjuvant chemotherapy. This in turn suggests that the increment in chemotherapy doses could result in higher radiosensitivity of the primary tumor and improve rates of complete clinical response. Recently, Gerard et al. reported that modifications in both radiotherapy and chemotherapy regimens have been implemented, and the results seem to support the idea of improved tumor response secondary to radiotherapy dose escalation [32]. These authors suggest that there are slightly improved complete pathological response rates in the group with an increased radiotherapy dose, with oxaliplatin offering no advantage over capecitabine alone as a radiosensitizer. The increased number of overall cycles of chemotherapy could have potentially positive benefits on long-term survival and decreased risk of late systemic relapses.
In a recent prospective study, the long-term results of patients who had complete clinical response following the extended chemoradiation regimen previously described, and managed nonoperatively using the “Watch and Wait” approach, were analyzed. Seventy consecutive patients with T2–4, N0–2M0, distal rectal cancer were studied. Neoadjuvant chemoradiotherapy included 54 Gy external beam radiation and 5-FU/leucovorin delivered in 6 cycles every 21 days, as previously described (Fig. 3.1). Patients were assessed for tumor response after all 6 cycles of chemotherapy, at 10 weeks from radiation completion. The assessment of response included clinical (digital rectal examination), endoscopic (proctoscopy), and radiological studies (MRI, ERUS imaging, and/or positron emission tomography (PET)/CT). Patients were considered as having an initial complete clinical response in the absence of residual ulceration, mass or significant rectal wall irregularity at the 10-week postradiotherapy follow-up. Radiological evidence of complete response was also required for inclusion in the “Watch and Wait” approach. Radiological features of complete response included the presence of residual low-signal-intensity areas (on MRI), absence of restriction to diffusion (on diffusion-weighted MRI) or absence of residual fluorodeoxyglucose (FDG) uptake within the rectal wall (on PET/CT). Patients with radiological evidence of residual tumor in the mesorectum and/or within the rectal wall were considered as incomplete responders, irrespective of clinical and endoscopic findings. Patients with incomplete clinical response were referred for immediate surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree