Outline
Open Maternal-Fetal Surgery, 776
Minimally Invasive Maternal-Fetal Surgery, 776
Ex Utero Intrapartum Therapy, 777
Conditions Amenable to Fetal Therapy, 778
Complicated Monochorionic Twins, 778
Myelomeningocele, 783
Congenital Diaphragmatic Hernia, 785
Congenital Pulmonary Airway Malformation, 788
Pleural Effusion, 789
Fetal Cardiac Interventions, 789
Lower Urinary Tract Obstruction, 790
Sacrococcygeal Teratoma, 791
Amniotic Band Syndrome, 791
Chorioangioma, 792
Summary, 793
Summary of Key Points
- •
Sonography has transformed the field of fetal therapy and serves as the widely available, necessary diagnostic and intraoperative guidance tool.
- •
The potential benefits of open maternal-fetal surgery must be weighed against the potential maternal and fetal risks, which have the potential of complicating the index pregnancy and subsequent pregnancies.
- •
Minimally invasive fetal surgery is associated with much lower maternal risks and is the preferred surgical approach for most fetal conditions amenable to in utero therapy.
- •
Monochorionic diamniotic twin pregnancies are associated with significant morbidity and mortality risks, and close surveillance with serial sonograms is warranted.
- •
Prenatal repair of myelomeningocele (MMC) is associated with improved neurologic outcomes and increased likelihood of an ability to ambulate without need for orthotics.
- •
The role of fetal therapy in severe congenital diaphragmatic hernia (CDH) remains under investigational protocols.
- •
The finding of fetal hydrops should be investigated carefully for underlying conditions that are potentially treatable in utero, such as anemia, congenital pulmonary airway malformation (CPAM), primary pleural effusion, sacrococcygeal teratoma (SCT), and chorioangioma.
- •
The field of fetal therapy is rapidly evolving. With further improvement of surgical equipment and prenatal diagnostic modalities, access to the fetal-placental unit via minimally invasive methods will likely become increasingly refined and provide a conduit for additional novel treatments of the fetus.
Intrauterine fetal transfusion for severe fetal anemia secondary to Rh hemolytic disease was the first invasive prenatal therapy performed in humans. It is difficult for current practitioners to imagine that initial efforts in the 1960s were performed in the absence of ultrasound guidance. Abdominal palpation and intrauterine and intraperitoneal instillation of contrast agent followed by fluoroscopy were used to perform the first fetal transfusions. Sonography has transformed the field of fetal therapy and serves as the ubiquitous diagnostic and intraoperative guidance tool.
An accurate detailed anatomic diagnosis is critical in determining the appropriateness of fetal surgical intervention. Fetal malformations that are not isolated or are associated with aneuploidy or a syndrome have generally been excluded as candidates for intervention. However, over the last few years, with the development of less invasive therapeutic options as well as increased accommodation for parental choice, this paradigm has shifted (e.g., pleuroamniotic shunting for effusions and hydrops in a fetus with trisomy 21). Great effort should be made to confirm both the accurate diagnosis of a malformation and to determine if it is isolated or if other, possibly subtle associated abnormalities are present. In addition to targeted obstetric sonography, ancillary techniques to ensure full and accurate assessment include fetal echocardiography, magnetic resonance imaging (MRI), and genetic studies such as karyotyping and chromosomal microarray analysis. Counseling of patients and their families regarding the fallibility of the process, explaining that abnormalities initially believed to be isolated may not ultimately prove to be so, is a must.
In addition to its role as the primary tool for fetal diagnosis, sonography is routinely used during fetal interventions, both for instrument guidance and to monitor the fetus. In the following sections, fetal conditions amenable to in utero therapy as well as the role of sonography in the diagnosis and treatment of various fetal abnormalities will be highlighted.
Open Maternal-Fetal Surgery
Maternal laparotomy/hysterotomy was historically the primary approach to fetal therapy ( Fig. 24-1 ). Over time there has been a shift away from this technique toward minimally invasive fetal interventions. The development of small-diameter endoscopes has enabled surgical access into the womb with less perturbation to the pregnancy. Still, maternal laparotomy/hysterotomy remains the primary surgical approach for in utero repair of MMC and is one treatment option for fetal lung lesions and teratomas.

Early experience with open maternal-fetal surgery was plagued by significant immediate postoperative complications, including maternal pulmonary edema related to use of multiple tocolytics, intraoperative blood loss, and refractory preterm labor. With refinement of surgical technique, anesthesia, and immediate postoperative care, the risks of open maternal-fetal surgery have decreased, although they still remain high compared to minimally invasive approaches. In 2011, the results of a randomized controlled trial comparing prenatal versus postnatal surgical repair of MMC (MOMS [Management of Myelomeningocele Study] trial) were published. In 78 patients who underwent open fetal surgery, the rate of maternal pulmonary edema was 6%, blood transfusion 9%, and preterm delivery before 30 weeks’ gestation 13%. The postprocedure rate of oligohydramnios was 21%, and the rate of placental abruption was 6%. With respect to maternal risk, uterine dehiscence at the time of delivery was noted in 10% of cases, with an additional 25% of cases described as having a very thin lower uterine segment. There were no maternal deaths or uterine ruptures. The procedure also has significant impact on future pregnancies, with a 28% risk of subsequent uterine dehiscence or rupture. All patients who undergo open maternal-fetal surgery require cesarean delivery before the onset of labor for the index pregnancy and all future pregnancies. As an American College of Obstetricians and Gynecologists Committee Opinion states, “Maternal-fetal surgery is a major procedure for the woman and her fetus, and it has significant implications and complications that occur acutely, postoperatively, for the duration of the pregnancy, and in subsequent pregnancies.”
Patients contemplating open maternal-fetal surgery undergo lengthy discussion about the risks of the procedure, with an emphasis on differentiating the risks to the mother, the fetus, and the pregnancy. The risks to the mother are similar to those for any major abdominal surgery, although in this case, there is no direct physical benefit to her. General anesthesia risks are relatively increased owing to the high levels of inhalational anesthetics required to achieve uterine quiescence. In addition, there are risks associated with aggressive tocolytic therapy and bed rest in pregnant women, who are in a hypercoagulable state. The risks to the fetus are primarily vascular instability and hypoperfusion intraoperatively, leading to organ injury or death, and prematurity due to postoperative complications. The risks to the pregnancy are primarily preterm labor and preterm premature rupture of membranes (PPROM) resulting in preterm delivery. Infectious complications are rare, except when PPROM leads to prolonged latency. As mentioned previously, an important additional discussion point is that all subsequent deliveries, as well as the index pregnancy, must be via cesarean delivery. Data regarding future fertility are reassuring, with no documented increased incidence of infertility. Another potential risk in subsequent pregnancies is placenta accreta; the site of a hysterotomy performed for fetal surgery in the second trimester is not the same as a lower segment cesarean delivery. Thus, placental implantation in a subsequent pregnancy is more likely to overlie the uterine scar, increasing the risk of accreta, and this factor must be considered in the sonographic evaluation of a subsequent pregnancy.
Sonography is used for guidance before the hysterotomy procedure in open maternal-fetal cases. Once general anesthesia and intubation of the patient have been initiated, and the sterile field is established, sonography is used to assess fetal lie, fetal location within the uterus, and position of the placenta. If needed, transabdominal manipulation is used to position the fetus such that the fetal surgical site is near the fundus. Laparotomy is then performed and the sonography transducer, covered in a sterile sleeve, is placed directly on the surface of the uterus. The location and edge of the placenta are identified, because this information is critical in determining where to safely enter the uterus. Generally, one wants to make the uterine incision as far from the placental edge as possible. Once the incision is made, and the amniotic fluid drains, the uterus shrinks. If the placental edge is too close to the uterine incision, there is a risk of bleeding and abruption, which cannot readily be controlled and, if significant, will usually necessitate immediate delivery for maternal safety.
Sonography is also used for transuterine monitoring of the fetal heart during the surgical repair of the MMC ( Fig. 24-2 ). Following completion of the fetal intervention, the membranes and myometrium are closed with several layers of suture. A catheter is left in the uterine cavity to allow lactated Ringer solution to be infused together with antibiotics. Sonography is used to determine the volume of “amniotic” fluid instilled to achieve a low-normal level, aiming to minimize stress on the suture line.

Postoperative management generally involves 24 hours of intravenous tocolysis with magnesium sulfate as well as maintenance with oral indomethacin for a total of 48 hours. Antibiotic “prophylaxis” is continued for 24 hours. Typically, ultrasound monitoring to evaluate the fetus, amniotic fluid volume, cervical length, and ductal patency is performed daily. Hospital discharge generally occurs 4 to 5 days after surgery. If the patient is stable without complications, long-term monitoring with follow-up sonograms usually involves weekly evaluation.
Minimally Invasive Maternal-Fetal Surgery
Minimally invasive fetal surgery generally refers to all invasive fetal interventions performed without the need for hysterotomy. Depending on the indications and goals of the procedure, minimally invasive fetal therapy may be performed under ultrasound guidance alone, such as needle or shunt placement procedures, or may require a combined approach with both sonography and endoscopy, also known as operative fetoscopy ( Table 24-1 ).
| Condition | Procedure |
|---|---|
| Needle and Shunts | |
| Alloimmunization | Transfusion |
| Bronchopulmonary sequestration | Interstitial ablation of feeding vessel |
| Congenital pulmonary airway malformation | Thoracoamniotic shunt or sclerotherapy |
| Critical aortic stenosis | Balloon valvuloplasty |
| Iatrogenic preterm premature rupture of fetal membranes | Amniopatch |
| Lower urinary tract obstruction | Vesicoamniotic shunt |
| Pleural effusion | Thoracoamniotic shunt |
| Pericardial teratoma with effusion | Pericardioamniotic shunt |
| Symptomatic polyhydramnios | Amnioreduction |
| Operative Fetoscopy | |
| Amniotic band syndrome | Lysis of constriction bands |
| Chorioangioma | Ablation of feeding vessels |
| Congenital diaphragmatic hernia | Tracheal occlusion |
| Myelomeningocele | Fetoscopic patch or closure |
| Twin reversed arterial perfusion sequence | Umbilical cord occlusion |
| Twin-twin transfusion syndrome | Laser photocoagulation of communicating vessels |
| Vasa previa (type II) | Laser occlusion of vasa previa |
| Sacrococcygeal teratoma | Interruption of vascular supply |
Ultrasound-guided needle interventions include intrauterine transfusion (IUT) and amnioreduction. Shunts are used when indicated for chronic drainage of abnormal, fluid-filled fetal cavities, organs, and cysts. A wide variety of fetal conditions have been surgically managed via operative fetoscopy. This technique, which utilizes a 3.3-mm or smaller endoscope, is associated with significantly lower risk of preterm birth, neonatal morbidity, and maternal morbidity as compared to open fetal surgery. There is a lower rate of preterm labor and of PPROM. The length of hospitalization and time until return to normal maternal activity are also shorter. In addition, patients who have undergone minimally invasive fetal therapy do not require cesarean delivery for the index pregnancy or for future pregnancies.
Minimally invasive procedures are performed under local or regional anesthesia. Depending on the gestational age and the protocols of the medical center, the procedure may be performed in the surgical operating room, labor and delivery unit, or ultrasound examination suite. Operative fetoscopy requires simultaneous utilization of ultrasound and fetoscopic imaging. Access to the amniotic cavity is achieved with thin-walled semiflexible plastic cannulas and either a plastic blunt-ended obturator using the Seldinger technique or sharp trocars. Specifics have been reviewed in several texts. Ultrasound imaging is used to identify an appropriate entry point and then to direct the instrument into the amniotic cavity, avoiding the placenta and the fetus and maternal organs such as bowel and bladder. The obturator/trocar is replaced by the fetoscope. Sonography is still used to direct the scope within the uterus, because its field and depth of view can be relatively limited. At the conclusion of the procedure, the sheath is removed. As the puncture is only 1 to 3 mm in size, the uterine wall spontaneously closes with minimal risk of amniotic fluid leak from the insertion site. In many cases, little or no tocolytic medication is needed, and patients are generally discharged from the hospital within 24 hours following the procedure.
Ex Utero Intrapartum Therapy
The ex utero intrapartum therapy (EXIT) procedure is a specialized surgical procedure performed at the time of delivery. It was first developed in the early 1990s to permit the controlled reversal of tracheal occlusion performed in utero for the treatment of severe CDH ( Fig. 24-3 ). This procedure is utilized in cases in which prenatal ultrasound findings predict significant neonatal airway compromise, resulting from lesions such as large oral or neck masses, or congenital high airway obstruction syndrome (CHAOS). Maternal general endotracheal anesthesia with high levels of an inhalational agent is provided to maintain uterine quiescence. The fetal head and neck are delivered through a low transverse uterine incision, protecting the umbilical cord and maintaining uteroplacental blood flow for an extended period of time. This allows for the performance of a surgical procedure or lifesaving intervention while the fetus continues to be oxygenated and ventilated through placental support. The EXIT procedure has been used to perform fetal bronchoscopy, laryngoscopy, tracheostomy, cannulation for extracorporeal membrane oxygenation (ECMO), and even resection of a lung mass or lobectomy.

Once the uterus is exposed, intraoperative ultrasound assessment is required to confirm placental location and fetal position. The hysterotomy is performed, avoiding placental margins along the lower uterine segment. Preventing complete decompression of the uterus is important to reduce the likelihood of placental separation. This can be achieved by keeping the lower part of the fetal body within the uterus and by performing continuous amnioinfusion with a catheter. The EXIT procedure is not a simple variation of a cesarean delivery and should be carried out in specialized centers. The presence of a complete multidisciplinary team is required to perform appropriate preoperative evaluation, including discussion of the fetal diagnosis, counseling, and planning for the EXIT procedure. The intraoperative team includes an anesthesiologist, maternal-fetal medicine specialist, pediatric surgical subspecialist(s), and operating room nursing assistants. Neonatologists and a second complete operative team should be prepared to receive the newborn in the event that corrective measures performed while still on umbilical circulation are unsuccessful.
Conditions Amenable to Fetal Therapy
Complicated Monochorionic Twins
Monochorionic twin gestations are associated with a high risk of significant perinatal morbidity and mortality as compared to dichorionic twins and singletons. This increased risk is partially attributed to the unique placental vascular communications present in monochorionic twin gestations. Because the circulatory systems communicate, the death of one monochorionic twin may result in acute hypotension in the survivor; this hemodynamic instability may result in subsequent death or injury to the co-twin. A systematic review of 28 primary studies, including over 4500 twin pregnancies with in utero demise of one twin, reported that the rate of monochorionic versus dichorionic co-twin demise was 12% versus 4%, and the rate of neurologic abnormality in the surviving co-twin was 18% versus 1%, respectively. Placental vascular communications are also thought to be important factors in the development of twin-twin transfusion syndrome (TTTS), twin anemia-polycythemia sequence (TAPS), and twin reversed arterial perfusion (TRAP) sequence.
Placental share is an additional factor with significant impact on perinatal outcomes in monochorionic twins. Marked unequal partitioning of the placental mass may be manifest clinically by selective fetal growth restriction (sFGR). Finally, the risk of structural anomalies is increased in monochorionic twins, including in particular those involving the central nervous system as well as cardiac and gastrointestinal tract abnormalities. Over 80% of such anomalies are discordant (only one twin affected). Some of these anomalies are associated with a high risk of fetal demise if managed expectantly. Even without the previously described complications, monochorionic twins are at an increased risk of neurodevelopment impairment. In one study, Ortibus and colleagues prospectively followed 136 monochorionic twin pregnancies from the first trimester through infancy. After excluding pregnancies complicated by TTTS, discordant growth, intrauterine fetal demise (IUFD), and prematurity (birth before 32 weeks’ gestation), the authors found that the 160 remaining infants had a 7% risk of neurodevelopmental impairment and a 0.6% risk of cerebral palsy.
Complications affect approximately 20% of monochorionic twins, and, thus, accurate diagnosis and close surveillance of these pregnancies are recommended. Chorionicity should be established as early as possible in the pregnancy. First and early second trimester ultrasound examination has been shown to be highly accurate in determining chorionicity and should be performed to allow for appropriate triage and management of the pregnancy. Close surveillance of monochorionic twins should include frequent sonograms, typically performed every 2 weeks, to monitor amniotic fluid volumes and fetal bladders. Cervical length assessment and Doppler interrogation should be performed, if necessary. If TTTS, TAPS, TRAP, sFGR, or discordant structural anomalies are suspected, the patient should be referred to an experienced center.
Twin-Twin Transfusion Syndrome
TTTS occurs in approximately 10% of monochorionic twins. Most experts believe that TTTS develops in monochorionic twin pairs as a result of unbalanced net flow of blood through vascular communications in the shared placenta. A series of pathophysiologic changes ensue from the net shunting of blood from one twin (donor) to the other twin (recipient), resulting in hypovolemia and oliguria in one, and volume overload and polyuria in the other ( Fig. 24-4A ). These hemodynamic derangements result in a cascade of physiologic changes leading to donor twin oligohydramnios, recipient twin polyhydramnios, and characteristic anatomic and arteriovenous flow alterations that can be identified by sonography with Doppler. Without intervention, early severe TTTS has a very high rate of perinatal injury or death.

The diagnosis of TTTS is based on the following in utero ultrasound-based criteria: (1) monochorionicity and (2) single maximal vertical pocket (MVP) of amniotic fluid less than 2.0 cm on one side of the dividing membrane, and MVP of more than 8.0 cm on the other side. Once the diagnosis has been established, the syndrome may be staged according to the Quintero staging system ( Table 24-2 ). The establishment of diagnostic criteria and the classification of TTTS by Quintero and associates. have allowed researchers to compare outcome data following various therapeutic interventions and to predict perinatal survival after treatment.
| Stage | Polyhydramnios/Oligohydramnios * | Persistently Empty Bladder in Donor | Abnormal Doppler † | Hydrops | Demise |
|---|---|---|---|---|---|
| I | X | ||||
| II | X | X | |||
| III | X | X | X | ||
| IV | X | ||||
| V | X |
* Polyhydramnios is defined as deepest vertical pocket 8 cm or greater; oligohydramnios is defined as deepest vertical pocket 2 cm or less.
† At least one of the following: (1) umbilical artery absent/reserved end-diastolic flow, (2) absent/reversed end-diastolic flow in the ductus venosus, (3) pulsatile umbilical venous flow.
The perinatal mortality rate of expectantly managed TTTS has been reported to be as high as 95%. Of the various treatment approaches that have been considered for TTTS, those with the best-reported outcomes are serial amniocenteses and selective laser photocoagulation of communicating vessels (SLPCV) via operative fetoscopy ( Fig. 24-4B ). Serial amniocentesis (or amnioreduction) involves drainage of amniotic fluid from the polyhydramniotic recipient sac using vacuum-assisted devices attached to an 18- to 20-gauge spinal needle. The volume of amniotic fluid that should be withdrawn has not been standardized, although usually the MVP of amniotic fluid in the recipient sac is reduced to approximately 8 cm or less. Serial amniocenteses serve to significantly reduce the amount of amniotic fluid in the recipient sac, thereby diminishing overall uterine distention.
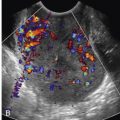
SLPCV is performed via operative fetoscopy, allowing direct visualization of the vessels on the placental surface. Once mapping of vascular communications has been performed, those vessels are occluded using laser energy ( Figs. 24-5 and 24-6 ). The basic principle of SLPCV is that the causative inter-twin vascular communications are ablated, thus treating the underlying pathogenic cause of this syndrome. With laser surgery, the circulations in a shared placenta are divided, functionally converting it to a dichorionic twin pregnancy, with improved perinatal outcomes.


After the introduction of laser ablation as a treatment option for TTTS, the Eurofetus group conducted a randomized controlled trial that compared serial amniocentesis to SLPCV. Pretrial power analysis projected the need for a total of 172 patients to demonstrate a significant difference in outcomes between the two treatment arms. However, after enrollment of 142 patients, interim analysis revealed that the perinatal survival rate of at least one twin was 51% (36/70) in the amnioreduction arm versus 76% (55/72) in the laser therapy arm. Based on this statistically significant difference, the study was halted. In addition, in the laser group, pregnancy duration was approximately 4 weeks longer (33 weeks vs. 29 weeks, P = 0.003). The amnioreduction group had a significantly higher rate of neurologic complications (14% vs. 6%, P = 0.02), and this difference persisted at the 6-month follow-up evaluation.
Most subsequent studies have confirmed that SLPCV is the optimal treatment for TTTS. Intervention with laser surgery has resulted in survival rates for at least one twin ranging from 65% to 93% and dual survival rates ranging from 18% to 62%. Although several factors may influence perinatal outcomes in laser-treated TTTS patients, one important consideration is disease severity. Knowledge of perinatal outcomes of TTTS pregnancies treated with laser, stratified by Quintero stage, may facilitate patient selection and counseling. In a series of 682 consecutive cases of TTTS treated via SLPCV, there was a 91% perinatal survival rate of at least one twin and 67% survival rate of both twins. Of note, perinatal survival of at least one fetus has been reported to be independent of Quintero stage, whereas dual twin survival does appear to differ based on stage. This is predominantly because stage III pregnancies are associated with decreased donor twin survival ( Table 24-3 ), particularly in those with growth restriction of the donor. These pregnancies are nearly twice as likely as those without growth restriction to suffer in utero demise of the donor. The average gestational age at delivery following laser surgery is approximately 33 weeks, with 10% delivering between 24 and 28 weeks’ gestation. The laser surgical technique has undergone recent modifications that may further improve perinatal outcomes.
| Stage I ( n = 114) | Stage II ( n = 177) | Stage III ( n = 328) | Stage IV ( n = 63) | |
|---|---|---|---|---|
| At least 1 survivor | 92% | 93% | 88% | 92% |
| Dual survival | 79% | 76% | 59% * | 68% |
* Decreased dual twin survival in Quintero stage III patients predominantly from decreased donor twin survival in cases complicated by severe growth restriction.
The surgical goal of SLPCV is to completely occlude all placental vascular connections between the twin pair. A missed or inadequately lasered vessel, referred to as a residual anastomosis (RA), may be associated with adverse perinatal outcomes such as persistent or reversed TTTS or TAPS. Pathologic confirmation of complete ablation of vascular communications can be ascertained via ex vivo placental injection studies in cases without placental maceration related to IUFD. RA after SLPCV has been reported to be as low as 4% to 5%, although some studies have reported frequencies of RA ranging from 32% to as high as 75% of cases. In a 2014 trial investigating laser coagulation of the entire vascular equator, the rate of RA was still 19%. The wide range of RA rates in these studies has been attributed to the laser technique employed and to the method of placental evaluation.
Despite evidence that SLPCV improves neonatal survival and reduces morbidity, cerebral injury is not fully prevented. Periventricular infarction, hemorrhagic injury, and ischemic white matter damage have been detected prenatally in fetuses treated with laser surgery for TTTS. The incidence of cerebral damage may be underestimated, because neurodevelopmental impairment and intellectual disability may only manifest several months or years after birth. Thus, a major concern for clinicians and parents is the rate of neurologic impairment in survivors. A systematic review found that the risk of neurodevelopmental impairment after laser therapy for TTTS is about 11%. Cerebral palsy was the most frequent diagnosis, accounting for about 40% of abnormal neurologic outcomes. In a cohort of 100 TTTS survivors treated with in utero laser surgery, formal neurodevelopmental testing, performed at 2 years of age, revealed normal range mean Battelle Developmental Inventory, 2nd edition (BDI-2) scores of 101.3 (standard deviation [SD] = 12.2) for the entire cohort. The overall rate of neurodevelopmental impairment, defined as blindness, deafness, cerebral palsy, or BDI-2 score less than 70, was 4%.
Selective Fetal Growth Restriction
sFGR, defined as one twin with an estimated fetal weight (EFW) below the 10th percentile for gestational age, is reported to occur in at least 10% of monochorionic twin pairs. It is important to note that significant growth discordance with or without amniotic fluid discordance is not required for the diagnosis of sFGR. sFGR appears to be distinct from TTTS, although there may be significant overlap in these conditions. It is believed that sFGR develops because of unequal placental share, with the sFGR fetus perfusing a smaller portion of the single shared placenta.
Although disproportion in placental territories allocated to monochorionic twin pairs appears to be the primary contributor to sFGR, the presence of vascular communications plays an important role in perinatal outcome. There is increased likelihood of spontaneous in utero demise of the sFGR fetus, which may result in concomitant demise or severe neurologic handicap of the co-twin. Following demise of the sFGR twin, sudden redistribution of blood flow via placental vascular connections can lead to acute hypotension in the surviving co-twin. Thus, the larger (appropriately grown for gestational age, AGA) twin may face an increased risk of neurodevelopmental impairment related to co-twin demise or prematurity. One observational study monitored 84 consecutive monochorionic twin pairs with discordant amniotic fluid that did not meet criteria for TTTS and showed a 60% (23/38 fetuses) overall survival rate in the subgroup that met criteria for sFGR. In another study of 17 pregnancies with sFGR that were managed expectantly, the survival rate of the sFGR twin was 59%. The rate of short-term intracranial injury (including periventricular leukomalacia, intraventricular hemorrhage, and ventriculomegaly) in this group of patients was 14%. In a separate review, the overall incidence of severe cerebral injury in survivors of pregnancies complicated by sFGR was found to be approximately 8%. Factors associated with poor neurologic outcomes included abnormal umbilical artery Doppler waveforms, intrauterine co-twin demise, and earlier gestational age at birth.
The understanding of the pathophysiologic changes of sFGR has advanced significantly in recent years. A staging system based on umbilical artery Doppler findings was proposed by Gratacos and colleagues in 2007 and is now used by some centers. The authors classified monochorionic twin pregnancies complicated by sFGR into three categories based on umbilical artery Doppler waveforms of the sFGR twin, as follows: type I with persistent forward diastolic flow; type II with persistent absent or reversed end-diastolic flow (AREDF); and type III with intermittent absent or reversed end-diastolic flow (iAREDF). These three groups are associated with different patterns of placental anastomoses and with different clinical courses, which can influence prenatal monitoring and management.
In type I sFGR, the median age at diagnosis is approximately 20 weeks’ gestation. In utero deterioration is not common in this group. Once this diagnosis is made, umbilical artery Doppler findings in the sFGR twin usually remain normal until delivery. It is reasonable to maintain close surveillance with weekly sonograms with Doppler, and to escalate follow-up as necessary. There appears to be no benefit from fetal intervention in this group, and it is reasonable to plan delivery at 34 to 35 weeks’ gestation in the absence of obstetric indications for earlier delivery.
At this time, the optimal treatment strategy for monochorionic twin pregnancies complicated by sFGR type II, with persistent AREDF in the umbilical artery, is not clear. Expectant management requires close monitoring of the pregnancy with sonography or fetal heart rate monitoring, often in the hospital setting, from 24 weeks’ gestation until delivery, aiming to prolong the pregnancy yet intending to deliver the twins prior to demise of the growth-restricted fetus. The umbilical artery Doppler findings cannot be used to predict imminent fetal demise. Doppler interrogation of the ductus venosus (DV) appears to be the best adjunct for fetal surveillance, as is done in singleton pregnancies complicated by FGR. Observation of absent or reversed a-wave flow on DV waveforms should prompt the clinician to consider delivery or fetal intervention, depending on gestational age. If the DV waveform remains normal, the patient can be followed once or twice a week. Expectant management is associated with a very high (nearly 100%) rate of prematurity, with risk of neurologic injury to the AGA twin. Intervention may be considered in cases of type II sFGR, particularly when the diagnosis is made prior to viability. One therapeutic option is to perform operative fetoscopic ablation of all inter-twin vascular communications, thereby creating a “functionally” dichorionic twin gestation. The surgical intervention is similar to the laser technique used for treatment of TTTS, although the procedure may be technically more difficult. Quintero and coworkers performed a feasibility study that showed a trend toward decreased rate of neurologic deficits in the AGA twin (14% rate of neurologic injury in the expectantly managed group versus 0% in the SLPCV group). Chalouhi and associates found that SLPCV resulted in a survival rate of 52% in sFGR twins and 74% in AGA twins. As an alternative to fetoscopic SLPCV, umbilical cord occlusion (UCO) or radiofrequency ablation (RFA) of the sFGR twin has been offered. These techniques aim to protect the AGA twin from potential injury or death resulting from spontaneous in utero demise of the sFGR co-twin. These approaches, by definition, result in demise of the sFGR fetus. Selective reduction in these circumstances has resulted in survival rates of the AGA co-twin of 74%.
Type III sFGR is characterized by iAREDF in the umbilical artery of the sFGR twin. The umbilical artery Doppler waveform pattern displays cyclic changes during diastole, such that there is alternating positive AREDF. This unique Doppler waveform suggests the presence of a large arterioarterial (AA) anastomosis within the shared placenta, coursing between the twins’ cord insertion sites. Outcomes of pregnancies with evidence of type III sFGR may be difficult to predict. Unexpected IUFD has been reported in approximately 15% of cases, with sudden death documented hours after a normal Doppler ultrasound examination. Another study has reported that the AGA twin has an elevated risk of postnatal white matter lesions, even if the smaller twin is born alive. Thus, management of these pregnancies is challenging. Fetoscopic laser ablation of vascular connections has been attempted in this population, but the results were not favorable compared to expectant management. SLPCV could not be completed in over 10% of cases owing to technical difficulties, and overall perinatal survival rate was 64% in the laser group compared to 86% in those managed expectantly. However, it should be noted that the prevalence of leukomalacia in the larger infant was 1/17 (5.9%) in the laser group versus 4/28 (14.3%) in the expectantly managed group.
Late-onset sFGR, referring to the development of growth restriction of one twin after 26 weeks’ gestation, is another previously described subgroup of complicated monochorionic twin gestations. The prevalence of late-onset sFGR among monochorionic twin pairs has been reported by Lewi and colleagues to be 6.3% in a cohort of 208 pregnancies. Mean gestational age at delivery was 35 weeks and 38% of infants met postnatal diagnostic criteria for TAPS. There was one intrauterine death.
Twin Anemia-Polycythemia Sequence
TAPS is an atypical form of TTTS that also appears to be caused by a net transfer of blood from one monochorionic twin to the other. TAPS is characterized by large inter-twin hemoglobin differences in the absence of oligohydramnios and polyhydramnios. This condition may develop spontaneously (2-6% of monochorionic twins) or as a result of residual vascular communications following laser surgery (2-13% of monochorionic twins who have had laser). Unlike TTTS, which is likely due to more acute hemodynamic imbalances, the pathogenesis of TAPS has been attributed to chronic net transfusion from one twin to the other, presumably at a slow enough rate to allow for hemodynamic equilibration. TAPS may result in severe anemia in one twin and polycythemia in the other ( Fig. 24-7 ). Placental pathologic studies have shown that pregnancies complicated by TAPS have relatively few, small-caliber (<1 mm) arteriovenous communications as compared to larger arteriovenous communications seen in placentas from pregnancies affected by classic TTTS.

Although there is not consensus in the literature, the antenatal definition of TAPS usually requires that the middle cerebral artery (MCA) peak systolic velocity (PSV) measures greater than 1.5 multiples of the median (MoM) in the anemic twin and less than 1.0 MoM in the polycythemic twin, and that the pregnancy is not affected by oligohydramnios or polyhydramnios. An antenatal staging system has been proposed, and although it has not been validated clinically, it is useful to facilitate communication between practitioners. Stage 1 is defined as MCA PSV measurement greater than 1.5 MoM in one twin and less than 1.0 MoM in the other, without evidence of fetal compromise. Stage 2 is defined as MCA PSV greater than 1.7 MoM in one twin and less than 0.8 MoM in the other, also without signs of fetal compromise. Stage 3 requires fulfillment of stage 1 or 2 criteria, with additional finding of critically abnormal Doppler waveforms (defined as AREDF in the umbilical artery, pulsatile flow in the umbilical vein, or reversed a-wave flow in the DV) in the anemic twin. Stage 4 includes hydropic changes in the anemic twin, whereas stage 5 is defined as demise, preceded by TAPS, of one or both twins. Postnatal diagnostic criteria and classification, described by the same investigators, requires an inter-twin hemoglobin difference of more than 8 g/dL and at least one of the following: reticulocyte count ratio more than 1.7 and placental pathologic findings of only small (<1 mm diameter) inter-twin vascular communications.
Given the relatively low prevalence and lack of clinical awareness of this condition, the natural history of TAPS is unclear and decision making regarding antenatal treatment is controversial. Case series have reported expectant management with timed delivery, intrauterine fetal transfusion, and fetoscopic laser treatment. Favorable outcomes have been described in pregnancies with uncomplicated TAPS using expectant management or other conservative measures, particularly in those diagnosed in the third trimester. Spontaneous resolution of TAPS has also been reported. However, TAPS may progress and result in perinatal death or neurologic injury. Polycythemia can lead to vascular sludging, which may lead to cerebral venous thrombosis resulting in intraventricular hemorrhage and parenchymal infarction, and fetal anemia may affect cerebral oxygenation resulting in hypoxic-ischemic cerebral injury. Timed delivery, on the other hand, must be weighed against the risks of prematurity. Although survival rates of 75% can be obtained with expectant management, it has been suggested that treatment with IUT or laser surgery can increase survival to over 90%. A recent 2014 study has reported that neurodevelopmental impairment and cognitive delay were found in almost 20% of children surviving posttreatment for TAPS, with the lowest cognitive scores detected in the subgroup of TAPS survivors treated with IUT. It is not known whether survivors of spontaneously resolved TAPS face a similar risk for neurodevelopmental impairment or cognitive delay.
IUT has been suggested as a temporizing, symptomatic treatment of the anemic twin in a pair affected by TAPS. However, there is concern that transfused blood may worsen symptoms in the recipient (polycythemic) twin, which might then develop iatrogenic complications related to hyperviscosity, such as limb necrosis. Two cases have exhibited spontaneous placental vascular thromboses, possibly because of hyperviscosity in the polycythemic twin. Thus, IUT (without definitive laser surgery) may exacerbate the risk of vascular thromboses and other complications. The use of intrauterine partial exchange transfusion may help reduce the risk of severe polycythemia complications. On the other hand, partial exchange transfusion in the neonate has been associated with suboptimal coagulation with subsequent disseminated intravascular coagulation, so this procedure is not without risk.
Fetoscopic laser surgery is the only definitive treatment for TAPS. Laser surgery may be beneficial in pregnancies complicated by TAPS, particularly in high-risk cases in which additional ultrasound findings suggest fetal compromise. However, laser surgery may be technically more challenging in TAPS than in TTTS given the absence of polyhydramnios, absence of a “stuck” twin, slack inter-twin membrane, and difficulty visualizing the very small communicating vessels. Nevertheless, reports of fetoscopic laser treatment for TAPS have indicated that this intervention can be effective. Further research is needed to establish selection criteria for in utero treatment of TAPS, possibly based on the presence of coexisting amniotic fluid discordance; Doppler waveform abnormalities in the umbilical artery, umbilical vein, or DV; sFGR; hydrops fetalis; cervical insufficiency; or gestational age at onset.
Twin Reversed Arterial Perfusion Sequence
TRAP sequence affects approximately 1% of monochorionic twins, or about 1 in 35,000 pregnancies. The presence of direct artery-to-artery and vein-to-vein placental anastomoses allows one fetus (pump twin) to perfuse the other anomalous fetus, typically referred to as an acardiac fetus (perfused twin). The term acardiac fetus is at times a misnomer, as the perfused twin may rarely have a rudimentary heart. The perfused twin is supplied with blood flowing in a reversed direction (the umbilical artery delivers blood to the perfused twin and the umbilical vein carries blood away, toward the placenta). Because of the direct artery-to-artery anastomosis, the deoxygenated blood from the pump twin is carried to the perfused twin through that twin’s umbilical artery or arteries. The perfused twin, typically with no functional beating heart, has severe maldevelopment of cephalic, thoracic, and upper extremity structures, which is not compatible with postnatal survival.
Ultrasound diagnosis of TRAP sequence can be made in the first and early second trimesters, particularly with the aid of color and spectral Doppler techniques. Note that the lack of a beating heart within the perfused twin may be misinterpreted as co-twin demise. In some cases, the presence of a rudimentary heart may be confusing. In this setting, it is important to interrogate the cord insertion site at the anterior abdominal wall of the anomalous fetus to assess for reversal of flow direction in the umbilical artery ( Fig. 24-8 ).

The risk of pump twin demise is as high as 50% to 75% due to the hemodynamic burden and volume overload. Risk factors for poor perinatal outcome include polyhydramnios in the pump twin, critically abnormal Doppler waveforms, hydrops, or a large perfused anomalous twin measuring greater than 50% of the pump twin’s estimated weight. If at least one of these factors is present between 16 and 26 weeks’ gestation, the patient should be offered the option of intervention to achieve UCO of the perfused twin. In one study of 74 cases complicated by TRAP sequence, high-risk factors were defined as at least one of the following ultrasound findings: (1) abdominal circumference of the perfused twin equal to or greater than that of the pump twin, (2) polyhydramnios (maximum vertical pocket > 8 cm), (3) abnormal Doppler waveform in the pump twin, (4) hydrops of the pump twin, or (5) monoamniotic twins. The perinatal survival rate for the high-risk pump twins in those patients who did not elect to undergo UCO was 43%. Current reported perinatal survival rates of high risk pump twins who have undergone UCO is approximately 80% to 90%.
A variety of techniques have been used to achieve UCO of the acardiac twin. Fetoscopic UCO is one common approach, and can be performed using a variety of surgical techniques, such as umbilical cord laser photocoagulation, laser of the placental vascular communications, and suture ligation. Similar results can be achieved with bipolar electrocautery and intrafetal RFA ( Fig. 24-9 ). Each approach is particularly suited to a given clinical circumstance, and the UCO modality is usually dictated by technical considerations, availability of instrumentation, and surgeon preference.

Twins With Discordant Structural Anomalies
Monochorionic twins may be affected by discordant structural anomalies. Patients with monochorionic twins discordant for a severe congenital anomaly, particularly those at significant risk of in utero demise, should be offered the option of selective reduction by UCO. Treatment via intravascular potassium chloride (KCl) injection is the primary method for selective reduction in dichorionic twins but is contraindicated in monochorionic twin gestations for two reasons. First, vascular communications within the monochorionic placenta may serve as a conduit for passage of the injected agent to the co-twin. Second, following reduction, acute exsanguination or hypotension of the co-twin via the connections in the shared placenta can result in neurologic injury or death. Thus, in these cases, UCO can be performed in a similar fashion to that described for TRAP sequence. As there are potential complications, including injury to the co-twin, those monochorionic twin pregnancies with discordant anomalies for whom UCO is considered require careful assessment and counseling of the risks and benefits particular for each case.
Myelomeningocele
Spina bifida is one of the most common birth defects in the United States, occurring in approximately 1 in 1500 liveborn infants annually. Spina bifida occurs when the embryonic neural plate fails to complete its development and does not fully close along its length. Failure of the posterior neuropore to close can result in meningocele (only the meninges protrude through the spinal defect) or MMC (both neural and meningeal elements protrude). Both lesions are referred to by the generic term spina bifida, with MMC being the more common type. Infants born with MMC require neurosurgical closure within the first days of life, and face lifelong disabilities, including varying degrees of sensorimotor paralysis below the level of the lesion, as well as bladder and bowel incontinence, musculoskeletal deformities, and spinal cord tethering at the site of surgical repair. For most individuals with MMC, lifelong support, rehabilitation, and medical interventions are likely.
The combination of maternal serum alpha-fetoprotein (MSAFP) screening and routine use of obstetric ultrasound imaging in the second trimester has resulted in approximately a 90% detection rate of MMC. Sonography typically reveals spinal dysraphism with splayed vertebral arches. The spina bifida lesion may appear flat with no overlying sac or may have a thin-walled sac filled with cerebrospinal fluid covering the placode. The sac may contain neural elements that can be seen on sonography ( see Chapter 9 , Fig. 9-7 ). Search for associated intracranial ultrasound findings is critical for maximizing in utero sonographic detection of spina bifida. The cisterna magna may appear obliterated and the cerebellum banana-shaped because of downward herniation of this structure into the upper cervical spinal canal (Chiari II malformation). A characteristic lemon-shaped calvarium may be seen, with inward scalloping of the frontal bones. Dilatation of the lateral cerebral ventricles is another common finding. Finally, equinovarus (clubfoot) deformity of one or both lower extremities can be found in fetuses with spina bifida.
MMC leads to injury and loss of spinal cord tissue at and below the level of the lesion. Abnormal spinal cord development may explain some of the neurologic deficits associated with MMC. However, mounting evidence suggests that intrauterine trauma and toxic exposure to amniotic fluid may contribute to the newborn’s neurologic impairment. The “two-hit hypothesis” of spinal cord injury in MMC has been shown in a variety of animal models, including sheep, rhesus monkey, pig, rabbit, rat, mouse, chick, and duck. The majority of these experiments have involved surgically created MMC, in which a multilevel laminectomy and dural opening are performed midgestation. Later in pregnancy, repair of the lesion is performed in one set of animals, whereas the lesion is left unrepaired in the others. The control group is then compared to the study group. The theoretical advantage of fetal repair of MMCs is that the neural tube is covered and protected many months before the expected delivery date. The basis for anticipating improved neurologic function is that coverage and restoration of the dysplastic neural placode within the spinal canal isolate it from amniotic fluid and prevent ongoing injury.
In animal models, prenatal correction of iatrogenic MMC has led to the birth of offspring with preserved motor function and sphincter control. Reversal of cerebellar herniation has also been noted in sheep that underwent intrauterine operations. Mueli and colleagues surgically created a spinal cord lesion in fetal sheep at 75 days of gestation that simulated an MMC lesion. Following delivery, pathologic evaluation of the exposed spinal cord in the control group resembled a human spina bifida lesion. These animals were incontinent and had loss of sensation and motor function below the level of the lesion. Other animals with surgically created spina bifida lesions were treated using a myocutaneous flap at 100 days of gestation. These animals subsequently had near-normal motor function and bowel and bladder control. In another animal experiment, hindbrain herniation mimicking Chiari II malformation was surgically created prenatally by establishing leakage of spinal cord fluid at the level of the distal cord. Fetal repair subsequently reversed this hindbrain herniation.
Encouraged by results in animal models, investigators began to repair MMC in human fetuses. Owing to technologic constraints, the surgical approach adopted has been open in utero repair ( Figs. 24-10 and 24-11 ). This procedure requires general anesthesia of the mother to maintain uterine quiescence, maternal laparotomy, hysterotomy, and neurosurgical closure of the fetal MMC. In 1999, Tulipan and coworkers reported outcomes of 26 of their first 28 cases of in utero MMC repair in humans, comparing them to historic MMC control subjects treated with postnatal surgical repair. Intrauterine MMC repair was associated with reduced incidence of moderate to severe hindbrain herniation (4% vs. 50%) and reduced occurrence of shunt-dependent hydrocephalus (58% vs. 92%). However, the average level of lower extremity function closely matched the average anatomic level of the MMC lesion in both groups. Similar findings were noted by other investigators. In 2003, the National Institutes of Health (NIH) funded a multicenter randomized controlled trial of open in utero surgical repair of fetal MMC versus standard neonatal repair. The results of this MOMS trial were published in 2011. Inclusion criteria specified a singleton gestation with upper level of the MMC lesion between T1 and S1, evidence of hindbrain herniation, gestational age at surgery between 19.0 and 25.9 weeks, normal karyotype, absence of anomalies unrelated to the MMC, absence of severe kyphosis, and maternal body mass index less than 35. Prenatal repair resulted in reduced need for ventriculoperitoneal shunting at 12 months of age, decrease in the rate of hindbrain herniation at 12 months, doubling of the ability to ambulate without the assistance of orthotics, and level of sensorimotor function two or more levels better than expected based on anatomic level of the defect. However, open in utero repair was associated with significant risks, including higher rates of preterm birth, fetal intraoperative bradycardia, oligohydramnios, placental abruption, maternal pulmonary edema, and maternal transfusion and a 35% risk of uterine thinning or dehiscence.