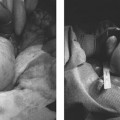
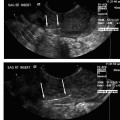
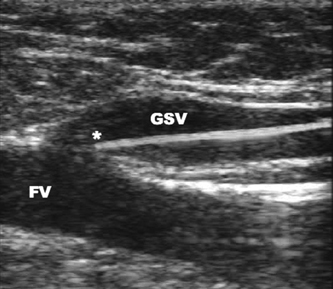
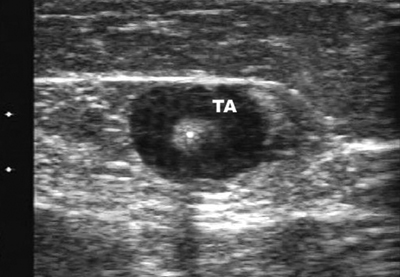
25 Saphenous Vein Ablation Robert J. Min Dr. Boné first reported on delivery of endoluminal laser energy in 1999.1 Since then, a method for treating the entire incompetent GSV segment has been described by Min and Navarro.2,3 Endovenous laser treatment, which received U.S. Food and Drug Administration (FDA) approval in January 2002, creates nonthrombotic vein occlusion by delivering laser energy directly into the vein walls. Lasers with wavelengths of 810 nm, 940 nm, 980 nm, 1064 nm, and 1320 nm have all been used with reported success. Contact between the laser fiber and vein wall is necessary to cause sufficient damage to the vein resulting in wall thickening with eventual contraction and fibrosis. Over the past 7 years, reports of impressive clinical success and low complication rates have made endovenous laser the treatment of choice for eliminating reflux in incompetent saphenous veins. Medical treatment of varicose veins may be contemplated when symptoms persist despite conservative methods such as gradient compression hose and exercise. Other indications may include treatment or prevention of complications arising from chronic venous hypertension such as bleeding, superficial thrombophlebitis, and damage to the skin. Relative contraindications for endovenous laser include nonpalpable pedal pulses, inability to ambulate, acute deep venous thrombosis (DVT), general poor health, or pregnancy. An additional relative contraindication to all catheter-based endovenous ablation techniques are nontraversable vein segments either due to thrombosis or extreme tortuosity. Fortunately, this is an uncommon finding and should be recognized on pretreatment venous duplex ultrasound (DUS) mapping. Prior to treatment, the abnormal venous pathways are mapped using Doppler ultrasound guidance and the veins to be treated are marked on the overlying skin with the patient standing. Additional important landmarks such as junctions, aneurysmal vein segments, and areas of significant blood inflow from tributaries or perforators should be marked on the skin. The patient is placed horizontal on the table, allowing full access to the areas that are going to be treated. In general, patients being treated for great saphenous vein (GSV), accessory saphenous vein (ASV), or thigh circumflex vein (TCV) reflux are placed supine or in the appropriate oblique position to expose the course of the target vein. When the small saphenous vein (SSV) is to be ablated, the patient is placed prone with his or her feet hanging off the end of the table to relax the calf muscle and popliteal fossa. Treating an incompetent Vein of Giacomini or multiple sources of venous reflux may require repositioning and reprepping multiple times. In almost all cases, the target vein is entered directly or access is gained via one of its principle tributaries. Tributary veins are more prone to venospasm and may be more difficult to access. Entry via a tributary should only be attempted if the vein is relatively straight and remains markedly enlarged in the horizontal position. The vein is usually punctured at, or just peripheral to, the lowest level of underlying reflux as determined by DUS. At this point, the vein diameter dramatically decreases just after it gives off a “refluxing escape” tributary and regains its competence. In many cases, GSV incompetence can occur segmentally. The reflux can completely spill out into a tributary vein, which then reenters the GSV at a lower level. In such a case, one should first access the lowest incompetent vein segment and treat all refluxing vein segments via one puncture, although more than one puncture is often necessary. The target vein is entered with a 19- or 21-gauge needle using real time US guidance and single wall technique. Utilizing reverse Trendelenburg positioning and keeping the procedure room warm until access is obtained helps minimize shrinkage of the target vein. Other ancillary procedures advocated by some practitioners to maximize vein size include a heating pad or a small amount of nitroglycerin paste at the access point. Non-GSV segments are particularly prone to venospasm and particular care must be taken when accessing a tributary vein or a non-GSV such as the ASV, SSV, or TCV. A 5F marked vascular sheath is inserted over a guide wire into the vein and passed through the entire abnormal segment and into a more central vein. A bare tipped laser fiber is inserted into the sheath. The sheath is then pulled back exposing a known length of the laser fiber tip and the fiber is fixed in place. Using US guidance, the sheath and fiber are withdrawn out of the deep veins and positioned within the superficial venous system at the junction as seen in Fig. 25.1. The fiber is left in this position during tumescent anesthesia administration and will be repositioned just prior to delivery of laser energy. Confirmation of the position can be made by direct visualization of the red aiming beam through the skin. Tumescent anesthesia is a form of local anesthesia delivery, which utilizes large volumes of dilute anesthetic solutions permitting anesthesia of large areas. Proper use of tumescent anesthesia should make endovenous laser painless without the need of intravenous sedation or general anesthesia. In fact, it can be argued that sedation adds risk to endovenous laser treatment by inhibiting patient feedback during the procedure as well as delaying immediate postablation ambulation. In addition to making endovenous laser painless, tumescent anesthesia is used to maximize efficacy and safety of treatment. Although venospasm may occur in portions of some accessed veins, proper delivery of tumescent fluid into the perivenous space is necessary to empty blood and to ensure compression of the vein around the laser fiber. Trendelenburg positioning or cooling the room to induce venospasm immediately before tumescent anesthesia will facilitate vein emptying. Contact between the vein walls and laser fiber tip will allow adequate transfer of laser energy to the target vein walls resulting in vein wall damage and subsequent fibrosis. Inadequate vein emptying with too much blood remaining within the vein will lead to nontarget heating. In the latter case if occlusion occurs, it will be the result of thrombosis with inevitable vessel recanalization. Fig. 25.1 Longitudinal ultrasound image at the saphenofemoral junction (patient’s head is to the left) during laser fiber positioning. The fiber and sheath are pulled out of the femoral vein (FV) and positioned within the great saphenous vein (GSV). The fiber tip (*) will be repositioned after tumescent anesthesia delivery and prior to laser firing. The surrounding cuff of tumescent fluid may also serve as a protective barrier and prevent heating of nontarget tissues, including skin, nerves, arteries, or the deep veins. Delivery of tumescent fluid in the proper plane can only be achieved with Doppler US guidance. This fluid is used to separate adjacent nontarget structures from the target vein. Some practitioners advocate injecting the fluid blindly or using highly pressurized devices to administer the tumescent fluid as is often done prior to liposuction. Although perhaps quicker, such systems offer less control compared with hand injection. For this reason, I prefer to deliver the tumescent anesthesia using hand pressure and a 1½ inch 25-gauge needle attached to a 20 mL syringe. For right-handed operators, the tumescent fluid is given from peripheral to central. Skin punctures are required every 3 to 5 cm until the proper perivenous tissue plane is located. Once this occurs, fluid will track more easily up and around the target vein and greater distances can be covered with each needle puncture. To treat a 45 cm segment of vein, ~100 to 150 mL of 0.1% lidocaine neutralized with sodium bicarbonate may be required. This mixture can be made by diluting 50 mL of 1% lidocaine in 440 mL of normal saline and adding 5 to 10 mL of 8.4% sodium bicarbonate. If it is anticipated that larger volumes of tumescent anesthesia will be necessary, a concentration of 0.05% lidocaine can be used effectively. These amounts of lidocaine are well within the safe doses of 4.5 mg per kilogram of lidocaine without epinephrine and 7 mg per kilogram with epinephrine. Although many practitioners choose to use lidocaine with epinephrine to maximize venospasm and minimize bruising, we achieve adequate and complete vein emptying utilizing plain lidocaine and avoid the risk of toxicity related to epinephrine. Following tumescent anesthesia delivery, ultrasound in the transverse plane is used to check for adequacy. A centimeter halo of fluid surrounding the target vein or separating the vein from the overlying skin is sufficient (Fig. 25.2). Proper delivery of tumescent anesthesia may be particularly important when performing endovenous laser of certain veins such as the SSV near the saphenopopliteal junction (SPJ) or the GSV below the knee due to their close proximity to nerves or arteries. Checking with color Doppler following tumescent anesthesia delivery can be useful when assessing adequacy of separation of arterial branches from the target vein. Fig. 25.2 Transverse ultrasound image of the vein walls compressed onto the laser fiber by a surrounding cuff of tumescent anesthesia (TA). Because the laser fiber can move during tumescent anesthesia administration, the laser fiber is repositioned prior to delivery of laser energy. For the GSV, the fiber tip is positioned 5 to 10 mm peripheral to the saphenofemoral (SFJ), usually at or just below a competent superficial epigastric vein. When treating the SSV, seeing the fiber tip can be difficult due to venospasm and the acute angle taken by the SSV as it dives to join the popliteal vein. Following delivery of tumescent anesthesia, the laser fiber tip may be more easily visualized with US (Fig. 25.3)
Patient Selection
Technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree