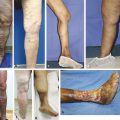
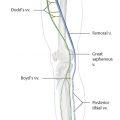
Venous sclerotherapy is the targeted injection of a chemical irritant into the lumen of a vein to produce inflammation, occlusion, and eventual fibrosis. A small amount of damage to the vein wall can induce intravascular thrombosis; however, in the presence of an intact endothelial lining, the vessel will usually recanalize over time. 1 In contrast, the goal of sclerotherapy is to cause irreversible complete endothelial cellular damage, full vessel wall destruction with permanent obliteration of the vascular lumen (endosclerosis), eventually resulting in a residual fibrous cord (endofibrosis). Sclerotherapy is indicated in the treatment of a wide range of abnormal veins—from small telangiectasias to incompetent truncal saphenous veins. The availability of multiple agents and variable drug concentrations and ability to modify agent composition (liquid or foam) combine to make sclerotherapy a powerful treatment modality in the management of varicose vein disease.
7.2 Indications
Injection sclerotherapy is indicated in a wide range of venous disease processes, including treatment of incompetent truncal and other large varicose veins, incompetent perforating veins, reticular and telangiectatic veins, and venous malformations. Although beyond the scope of this chapter, its use extends to treat other medical conditions, including submucosal esophageal and gastric varices, scrotal varicoceles, vulvar varicosities, hemorrhoids, hygromas, lymphatic cysts, and Baker’s cysts. 2
Liquid sclerotherapy is considered the treatment of choice for small reticular veins and telangiectasias—CEAP (clinical, etiology, anatomy, pathophysiology) class C1 (see Chapter ▶ 3, “The Clinical Exam,” for the CEAP classification system). 2 Foam sclerotherapy is also the treatment of choice in suitable CEAP class C2 varicose veins. 2 In addition, along with more established surgical and endovascular ablative techniques, studies have proven foam sclerotherapy to be a safe, effective, and affordable treatment for saphenous vein incompetence. 3, 4, 5
7.3 Contraindications
Absolute contraindications to injection sclerotherapy include known allergy to the proposed sclerosant agent, acute deep venous thrombosis, active infectious process involving the area to be treated, and prolonged immobility which increases the risk of postprocedure deep vein thrombosis (DVT). In addition, when the use of foam sclerotherapy is planned, the presence of a symptomatic right-to-left cardiac shunt should be considered an absolute contraindication due to the increased risk of paradoxical air embolism, a complication discussed later in this chapter. 2
Postsclerotherapy compression is important in reducing thrombophlebitis, and therefore, a patient’s inability to wear compression stockings (as exists with significant lower extremity arterial insufficiency) should be considered a relative contraindication. 6 Other relative contraindications should be considered on a case-by-case basis and include pregnancy, inability to ambulate, prothrombotic syndromes, and multiple drug allergies. More specifically, care must be taken in patients taking the estrogen receptor antagonist tamoxifen as the incidence of superficial thrombophlebitis is higher. 7 Also, patients taking the acetaldehyde dehydrogenase inhibitor disulfiram should avoid polidocanol [POL] because it contains ethyl alcohol (see later). 6 Finally, a thorough risk/benefit discussion should also be had with any patient who had neurologic symptoms following prior administration of foam sclerotherapy. 8
7.4 Sclerosing Agents and Mechanisms of Action
Sclerosants are designed to irreversibly damage the vascular endothelium but are also capable of damaging surrounding tissue. This endothelial destruction is dependent on the administered sclerosant dose and the contact time between the agent and the endothelial layer. The ideal sclerosing agent would cause rapid full-thickness vascular wall damage with minimal thrombus formation, be minimally toxic to adjacent structures, be hypoallergenic, and not result in hyper- or hypopigmentation of the overlying skin. Unfortunately, such an agent does not exist; however, the careful use of modern sclerosant medications in the appropriate concentration and form will usually result in a safe and effective treatment. Effective sclerotherapy is dependent on damage of the entire vessel wall, not just the endothelial layer. Rapid endothelial regeneration and migration can occur if the surrounding vascular smooth muscle cells are left intact, leading to eventual vessel recanalization. 9 In vitro work with the two most widely used detergent sclerosant agents (0.3% POL and 0.1% sodium tetradecyl sulfate [STS]) has demonstrated cell death in cultured endothelial cells within 15 minutes of incubation. 10 In vivo, endothelial cell death causes exposure of collagen fibers, which in turn activates the intrinsic blood coagulation pathway. As a result, thrombus generation always occurs to some degree following sclerotherapy but can be problematic, leading to excessive inflammation and eventual recanalization. Thrombus generated following full-thickness endothelial damage has different properties to conventional intravascular thrombus, including a higher white blood cell count and inflammatory infiltration of the vessel wall, making it strongly adherent. 11 It is important to stress that sclerosant agents do not mediate their effects through the generation of thrombosis but rather via direct vessel wall injury.
Studies have shown that patients receiving systemic anticoagulation medications had no reduction in treatment efficacy following foam sclerotherapy. 12 Similarly, the addition of heparin to the detergent sclerosant STS did not reduce its treatment efficacy. 13 Sclerosing agents can be broadly classified into three categories based on the mechanism of cell damage: detergent-based sclerosants, hyperosmotic agents, and chemical irritants. Agents are usually administered individually but may be combined for a particular clinical indication. 14
7.4.1 Detergent Solutions
In worldwide use since the 1930s, detergent-based agents are now the most popular sclerosant class in the treatment of varicose vein disease. Above a certain concentration, detergent molecules organize themselves into micelles, which, via a number of mechanisms including a process called protein theft denaturation, cause disruption of the cell surface membrane through interference of cell surface lipids. 15 Irreversible cell changes occur within minutes of contact. Agents include sodium morrhuate, ethanolamine oleate, and the two most widely used agents—STS and POL. Sodium morrhuate (Scleromate, Palisades Pharmaceuticals) and ethanolamine oleate (Ethamolin, QOL Medical) are agents which were in use prior to the establishment of the Food and Drug Administration (FDA) in the United States and were accordingly “grandfathered in” primarily for use in the treatment of esophageal varices. Their use in the treatment of lower extremity varicose veins is not routinely recommended given reported skin necrosis with extravasation of both agents and the relatively high incidence of severe anaphylactic reactions with sodium morrhuate. 1 Given the modern widespread use of STS and POL, we will focus on these agents in more detail.
Sodium Tetradecyl sulfate
STS (sodium-1 isobutyl-4-ethyloctyl sulfate; Sotradecol, Bioniche Pharma Group, Inverin, Co.; Thromboject, Omega Laboratories; Trombovar, Laboratoires Innothéra; Fibro-Vein, STD Pharmaceutical Products) is a long-chain fatty acid salt and has been in commercial use in the United States since before the establishment of the FDA in 1938. Accordingly, this agent was also “grandfathered in” and as a result it was never objectively evaluated for its role in venous reflux disease. Nevertheless, STS has been in widespread use for more than 60 years and has a proven track record in terms of both safety and efficacy. 15 It is commercially available in concentrations ranging from 0.2 to 3% and comes as a clear nonviscous fluid. STS is a powerful sclerosant agent and has been shown to produce maceration of the endothelium within 1 second of exposure. 16 STS is approximately two to three times as potent as POL at the same concentration. The maximal advised sessional dose varies between manufacturers, with a volume of 4 mL of 3% STS recommended by STD Pharmaceutical Products but up to 10 mL of 3% solution is recommended by Bioniche Pharma Group. 17, 18 Larger volumes may be appropriate at lower concentrations. For adverse reactions, see later.
Polidocanol
POL (laurel macro gel 400 laureth-9; Aethoxysklerol, Asclera Kreussler & Co., GMBH) is a synthetic long-chain fatty acid nonionic detergent with local anesthetic properties. It was originally marketed in 1936 as a topical anesthetic agent, but its use as a vascular sclerosant did not start until the 1960s. 1 It is marketed as an injectable sclerosant and is available in a number of different concentrations ranging from 0.25 to 4%. A dose limit of 2 mg per kg body weight is described (10 mL of 1% solution in a 50-kg patient). 15 However, other authors have reported higher volumes and concentrations with no ill effects. 19 It was approved by the FDA in 2010 for use in the United States in treatment of telangiectasias and reticular veins only. Although considered a weaker sclerosant agent than STS, POL is equally efficacious as a venous sclerosant with a generally better safety profile. It is painless to inject, and allergic reactions have rarely been reported. For adverse reactions, see later.
Detergent Foams
Agitation of liquid detergent sclerosant with a gas results in creation of foam. When injected into the vein lumen, this detergent foam has the ability to displace blood rather than mix with it and thus maximizes endothelial surface contact area and time, all with a lower required volume of liquid solution. It should be noted, however, that if the injected foam volume is too small for the vein diameter, the foam may simply float on the intravascular blood contacting only the nondependent endothelial surface. Cabrera et al first described the use “sclerofoam” in the 1990s, building on much earlier work from Foote in 1944 and Orbach and Petretti in 1950. 20, 21, 22 A simple technique to produce a durable foam was described by Tessari et al using a three-way connecter. 23 They found a ratio of one part liquid sclerosant to four parts air to be optimal in generating a dense, durable foam. Carbon dioxide, oxygen, and room air have all been used in foam generation, with no clear consensus on the optimal sclerosant to air/gas ratio. 24 As carbon dioxide is denser than room air, foam bubbles are smaller in size. This is advantageous as smaller bubble size translates to increase in surface area of the sclerosant and less blood mixing. CO2-based foam, however, degrades more quickly than room air and must be injected rapidly following generation. Some authors have shown that when high-volume foam administration is performed, the use of CO2-based foam appears to reduce early-onset reversible neurologic side effects when compared with room air. 25 Others have shown no difference in neurologic effects between air-based foam and that generated with a 30% O2/70% CO2 gas mixture when used at lower foam volumes. 26 Although there is no evidence-based limit for the maximum volume of foam administered per session, the European Consensus on Foam Sclerotherapy considered 10 mL as safe as an expert opinion. 8
7.4.2 Hyperosmotic Agents
Sclerosing agents in this class include hypertonic saline (HS), sodium salicylate (Saliject, Omega Laboratories), and formulations of dextrose and HS (Sclerodex, Omega Laboratories). Concentrated hyperosmolar solutions are thought to partially denature cell surface proteins through dehydration but require several minutes to exert their full effect, especially as full-thickness vein wall destruction is required. Studies have shown that endothelial cell death using HS occurs only after 3 minutes of contact time and treated endothelial cells do not detach into the vessel lumen as seen following administration of detergent-based sclerosant agents. 16 Accordingly, these agents are primarily suited for small vessels such as extremity telangiectasias and small reticular veins.
HS is a weak sclerosant in use since the 1920s. Currently, HS is only FDA approved for use in the United States as an abortifacient and accordingly its use is considered off label for venous sclerosis. The principal advantage of HS over detergent-based sclerosant agents is the absence of allergic reactions when given in its pure form. The major drawback of HS is pain on injection and the potential for extensive tissue necrosis with even a small amount of extravascular administration. 6 Administration has also been associated with severe muscle cramping. In addition, hyperosmotic agents cause erythrocyte hemolysis, which can lead to punctate hyperpigmentation. Other potential complications include hematuria, renal cortical necrosis, and hypertension. 6
Practitioners have described diluting the HS solution with lidocaine to vary the HS solution between 18 and 30% depending on vein caliber. 27 The role of HS in venous disease is now generally limited to injection of telangiectasias in patients with a documented allergy to detergent sclerosant agents. HS with dextrose formulations are believed to be less painful to inject and are associated with less muscle cramping. 28 Sodium salicylate is usually diluted with lidocaine to reduce pain but is again associated with muscle cramping and risk of necrosis if extravasation occurs. 6
7.4.3 Chemical Irritants
Agents in this category include denatured alcohol, polyiodinated iodine (Sclerodine, Omega Laboratories; Variglobin, Globopharm), and chromated glycerin (Sclérémo, Laboratories Bailleul; Chromex, Omega Laboratories) and act directly on endothelial cells, likely via disruption of intercellular binding proteins. Glycerin is a mild sclerosant that rarely causes hyperpigmentation or necrosis and is hypoallergenic in its pure form. 29 Although a direct chemical irritant, it likely also exerts an osmotic effect. Given its high viscosity, it can be difficult to inject through a narrow gauge needle and is often diluted with lidocaine. The addition of chromium increases the sclerosant potency and prevents hematuria, which can be seen with use of glycerin alone in high doses. 30 It has a very good side-effect profile; however, its use is cautioned in diabetic patients because there is a potential risk of hyperglycemia. 6
7.5 Sclerotherapy Technique
As with any medical intervention, a thorough informed consent is required outlining the risks and benefits of the proposed procedure. It is important to stress the realistic goals of treatment and to explain from the outset that short- and medium-term follow-up sessions may be required. Specific complications related to injection sclerotherapy should be discussed (see later). Increasing the concentration and volume of administered sclerosant will increase the likelihood of treatment success; however, the risk of complications also rises. Ideally, the minimum concentration and volume of agent should be delivered to cause irreversible vascular damage while sparing the surrounding tissues.
7.5.1 Liquid Sclerotherapy for Reticular Veins and Telangiectasias
Technique
Most practitioners advise against shaving the treatment area on the day of the procedure because this may lead to stinging on application of alcohol-based antiseptic solutions. Application of moisturizing cream is also discouraged as it renders the skin slippery and difficult to make taut during vein cannulation. 6 Photographs should be taken of the lower extremities to act as a baseline prior to initiation of a treatment regimen. The treated body part should be placed in a horizontal position as gravity-aided dilatation is not needed in treatment of reticular or telangiectatic veins (and may worsen intravascular thrombosis). It is very helpful to use an adjustable tilt table which allows positioning of the patient at a comfortable height for the operator but also to have the ability to place the patient in Trendelenburg’s or reverse Trendelenburg’s position as required. The area is cleansed using an alcohol wipe, which in addition to an antiseptic effect also renders the skin somewhat more transparent and may even cause a degree of vasodilatation. 6 Many operators find the use of magnification with or without polarized light greatly beneficial in visualization of small telangiectasias and reticular veins ( ▶ Fig. 7.1).
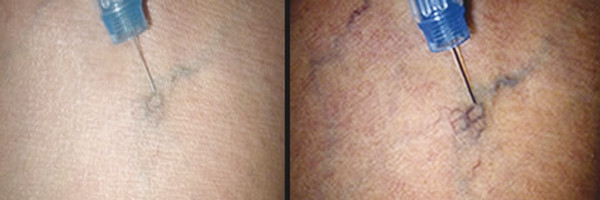
Fig. 7.1 Telangiectatic vein appearance using polarized light.
(Reprinted with permission from Syris Scientific.)
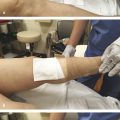
The use of small caliber (30 G) needles is advised to minimize trauma to the vein wall. Some authors advocate the use of even smaller (32–33 G) needles for very small vessels, but such needles tend to bend easily. 31 The needle is connected to either a 3 or 1 mL syringe, sometimes via a short segment of kink resistant connecting tubing. It should be remembered that much higher pressures are generated with use of small-volume syringes and care must be taken to avoid vessel injury and extravasation. As a general rule, the telangiectatic vessel should be injected slowly and with the least force required to fill the vessel lumen. Gentle tension should be applied to the skin to stabilize the target vessel. As telangiectasias lie within the dermis, it is important to be as superficial as possible. Most practitioners find it helpful to bend the needle slightly and to enter the vessel with the bevel facing upward, almost parallel to the skin surface ( ▶ Fig. 7.2). Some physicians advocate injection of a minute amount of air to confirm intravascular position prior to injection of sclerosant, the so-called air block technique. 32 Alternatively, aspiration of a small amount of blood and rapid linear blanching of the target vein on injection of sclerosant also indicates satisfactory intravascular position. Generalized, nonlinear whitening of the tissue or burning pain is indicative of extravascular position of the needle, and injection should be stopped immediately.
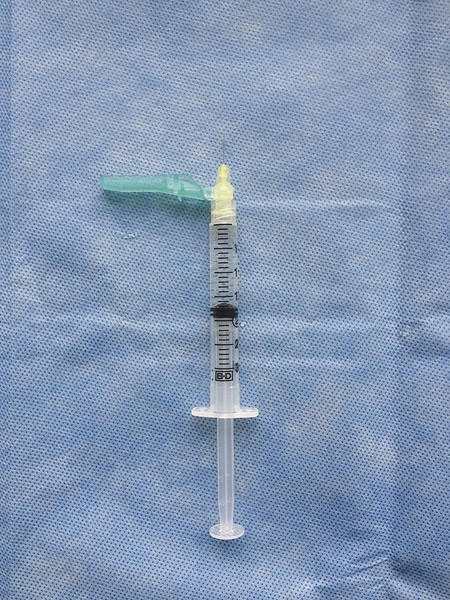
Fig. 7.2 Liquid sclerotherapy: 3-mL syringe and a 30-G needle with 140-degree angulation to aid cannulation of superficial telangiectatic veins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree