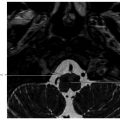
2 Sellar, Parasellar, and Clival Region The central skull base primarily develops from cartilage precursors, except for the most lateral portions of the greater wings of the sphenoid bone. Conversion of the mesenchyme into cartilage at the skull base begins around the 40th day of gestation. The various foramina of the skull base are present within this cartilaginous formation because the primitive nerves develop prior to chondrification and the cartilage develops around them. During the 5th week of gestation, the notochord passes into the basiocciput from the upper cervical vertebral bodies. It then passes obliquely through the basiocciput, exiting ventrally to come in contact with the primitive pharynx, and then back into the basisphenoid and terminates just caudal to the pituitary fossa at the dural margin.1 Chordomas and ecchordosis physaliphora are thought to arise from notochord remnants. The Rathke’s pouch, which gives rise to the anterior pituitary, arises from an invagination of the oral ectoderm from the roof of the stomodeum. It passes through the basal mesoderm just cephalad to the tip of the notochord to meet the precursor of the posterior pituitary, which forms from a diverticulum of the diencephalon.2 This tract is usually obliterated by later ossification, but can rarely persist postnatally (termed the craniopharyngeal canal) and contain ectopic anterior pituitary tissue.3 The body of the sphenoid bone is formed from fusion of the two lateral hypophyseal cartilages containing and surrounding the developing pituitary gland. More rostrally, the paired presphenoid cartilages fuse to become the most anterior portion of the sphenoid bone. Laterally, there is fusion between precursors of the lesser (orbitosphenoid) and the greater (alisphenoid) wings.1,2 As ossification progresses, most of the cartilage is obliterated. However, remaining cartilage can be found in various synchondroses and persists into adult life, most commonly in the foramen lacerum and petroclival junction. Skull base chondrosarcomas are thought to mostly arise from remnants of embryonal cartilage. Most of the central skull base is formed by the sphenoid bone. It is a structurally complex butterfly-shaped bone consisting of a central body (containing the sella turcica above and the sphenoid sinus below), two pairs of lateral extensions (greater and lesser wings), and one pair of inferior extensions (pterygoid processes; The sella turcica contains the pituitary gland and is a saddlelike bony formation on the upper surface of the sphenoid body ( The sphenoid bone contains a number of important neurovascular canals ( Table 2.1 Sphenoid anatomy important for transsphenoidal surgery
2.1 Embryological Development
2.2 Basic Anatomy Overview
 Fig. 2.1).
Fig. 2.1).
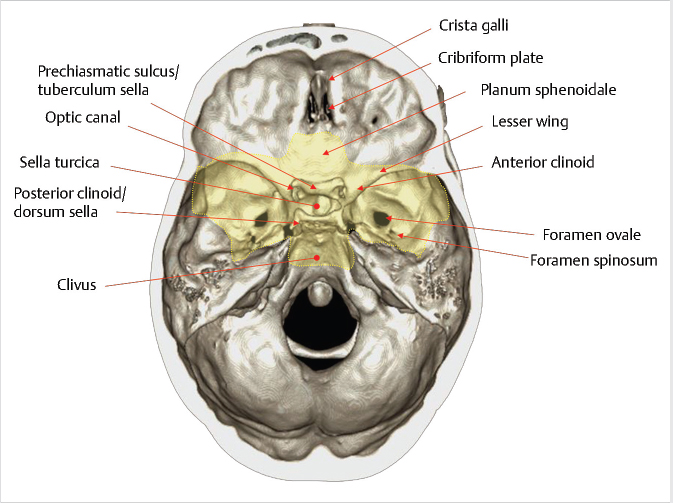
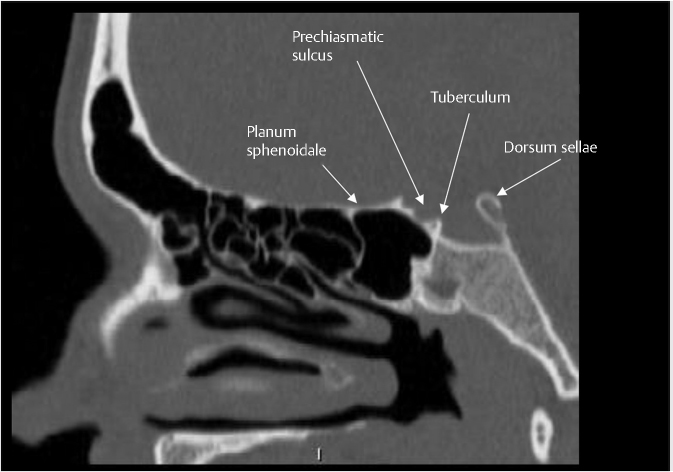
 Fig. 2.2). It is bounded anteriorly by the tuberculum sellae and posteriorly by the dorsum sellae. Anterior to the tuberculum sellae is the prechiasmatic sulcus and the planum sphenoidale. The sella turcica is surrounded by four bony projections, the anterior and posterior clinoid processes. The dorsum sellae is contiguous posteroinferiorly with the clivus through the spheno-occipital synchondrosis.
Fig. 2.2). It is bounded anteriorly by the tuberculum sellae and posteriorly by the dorsum sellae. Anterior to the tuberculum sellae is the prechiasmatic sulcus and the planum sphenoidale. The sella turcica is surrounded by four bony projections, the anterior and posterior clinoid processes. The dorsum sellae is contiguous posteroinferiorly with the clivus through the spheno-occipital synchondrosis.
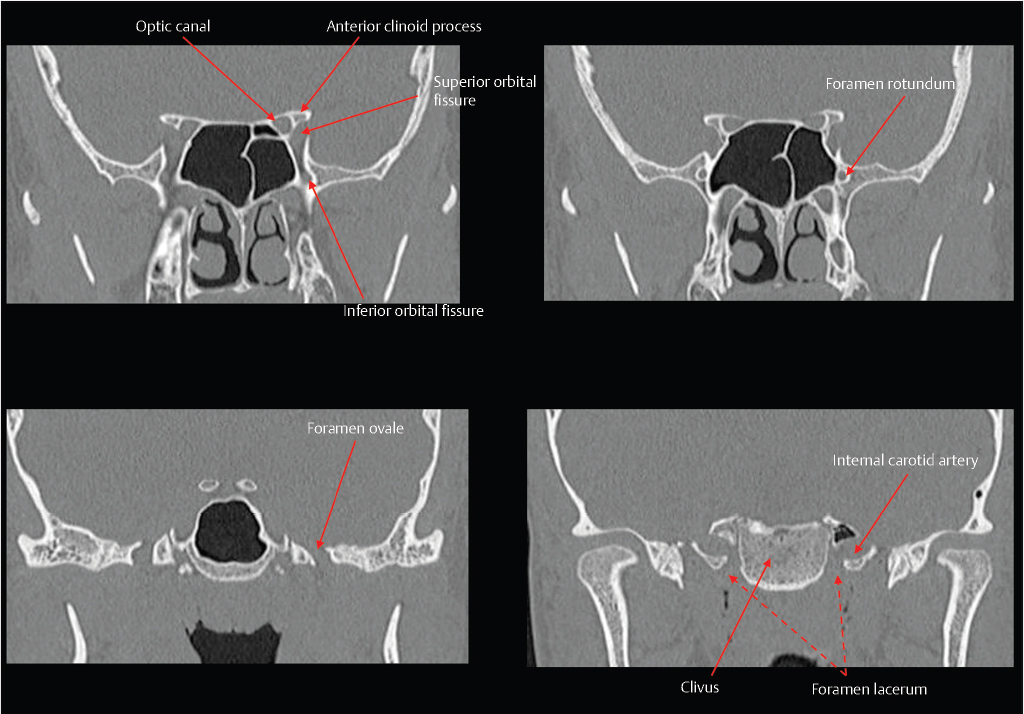
 Fig. 2.3). The superior orbital fissure is located between the greater and lesser wings, and transmits the superior ophthalmic vein and cranial nerves III, IV, VI, and V1. The optic canal is located above and is separated from the superior orbital fissure by the optic strut, and transmits the optic nerve and the ophthalmic artery. Other foramina near the junction between the sphenoid body and greater wing include the vidian/pterygoid canal (vidian artery and nerve), foramen rotundum (CN V2), foramen ovale (CN V3), and foramen spinosum (middle meningeal artery).
Fig. 2.3). The superior orbital fissure is located between the greater and lesser wings, and transmits the superior ophthalmic vein and cranial nerves III, IV, VI, and V1. The optic canal is located above and is separated from the superior orbital fissure by the optic strut, and transmits the optic nerve and the ophthalmic artery. Other foramina near the junction between the sphenoid body and greater wing include the vidian/pterygoid canal (vidian artery and nerve), foramen rotundum (CN V2), foramen ovale (CN V3), and foramen spinosum (middle meningeal artery).
Pneumatization pattern of the sphenoid sinus (sellar, presellar, conchal) Lateral pneumatization for transpterygoid approaches |
Presence of Onodi cell |
Position of the intersphenoid septum relative to the internal carotid artery or optic nerve |
Dehiscence of the bony covering around the internal carotid artery or optic nerve |
Vascular variants (such as aberrant ICA or persistent trigeminal artery) and aneurysms |
Presence of paranasal sinus inflammatory disease |
Abbreviation: ICA, internal carotid artery.
The sphenoid sinus is a variably pneumatized posterior extension of the paranasal sinuses. It is bordered by the ethmoid air cells anteriorly, clivus posteriorly, cavernous sinuses and cavernous internal carotid arteries laterally, sellae turcica and planum sphenoidale superiorly, and the nasopharynx inferiorly. There are several anatomic features related to the sphenoid that are important to assess when planning for surgery ( Table 2.1).
Table 2.1).
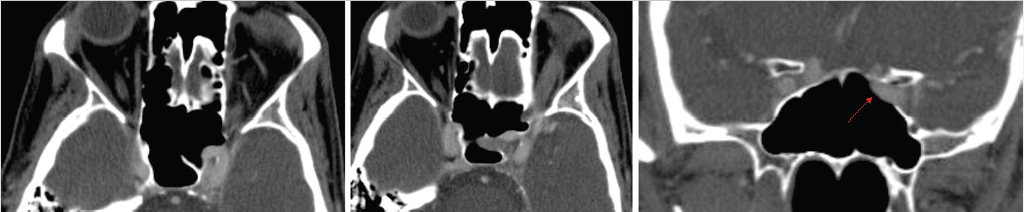
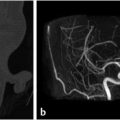
The sphenoid sinus is divided by complete and incomplete bony septations in various orientations. These septations may attach to the carotid canal, which is important because care must be taken to avoid carotid artery injury when removing these septations ( Fig. 2.4). It is also important to recognize the presence of any bony dehiscence of the carotid, as it protrudes centrally into the sphenoid (
Fig. 2.4). It is also important to recognize the presence of any bony dehiscence of the carotid, as it protrudes centrally into the sphenoid ( Fig. 2.5). This would make the vessel more vulnerable to injury.
Fig. 2.5). This would make the vessel more vulnerable to injury.
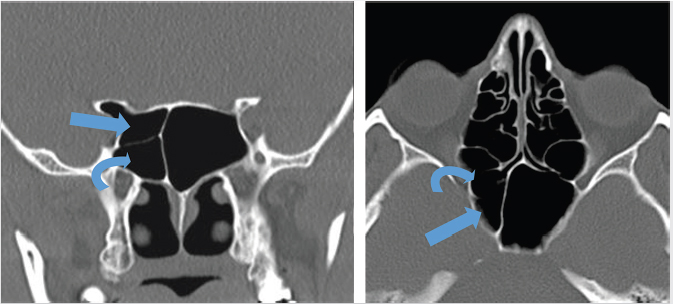
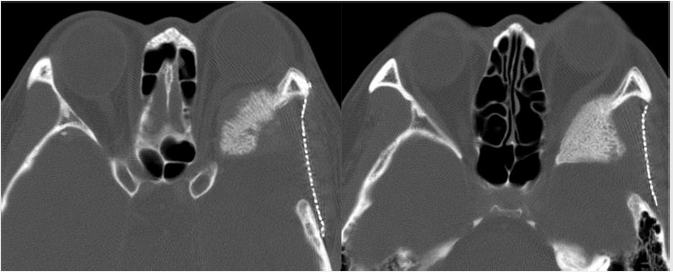
The Onodi cell is a posterior ethmoidal cell that pneumatizes and extends posterior and superior to the sphenoid sinus ( Fig. 2.6). This air cell is present in approximately 10% of the Western population. On coronal computed tomography (CT), a clue to the presence of the Onodi cell is visualization of a horizontal or cruciform septation, which represents the wall between the Onodi air cell superiorly and the sphenoid sinus inferiorly. As a result of its location, the optic nerve and/or the internal carotid artery (ICA) may be encompassed by it and be vulnerable to injury during surgery. The surgeon may also become disoriented with regard to the usual anatomy if this is not recognized.
Fig. 2.6). This air cell is present in approximately 10% of the Western population. On coronal computed tomography (CT), a clue to the presence of the Onodi cell is visualization of a horizontal or cruciform septation, which represents the wall between the Onodi air cell superiorly and the sphenoid sinus inferiorly. As a result of its location, the optic nerve and/or the internal carotid artery (ICA) may be encompassed by it and be vulnerable to injury during surgery. The surgeon may also become disoriented with regard to the usual anatomy if this is not recognized.
Fig. 2.4 Sphenoid sinus septations attaching to the carotid artery canal. Care should be taken when surgically removing these septations to avoid carotid artery injury.
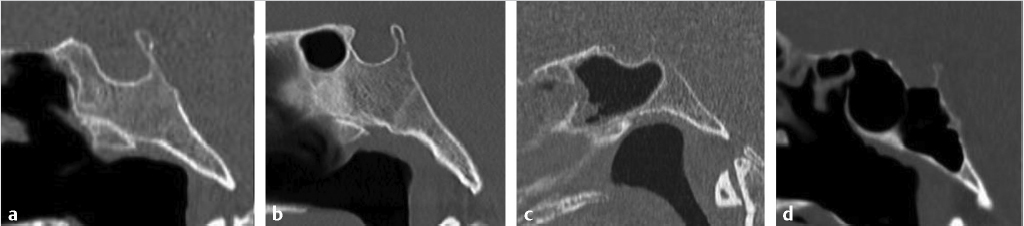
Pneumatization of the sphenoid sinus is highly variable and can extend as far as the clivus and foramen magnum inferiorly, and the sphenoid wings laterally. The traditional classification by Hamberger et al4 and Hammer and Radberg5 describes three types of sphenoid pneumatization according to the proximity of the aerated cavity to the sellar floor: sellar (80%), presellar (17%), and conchal (3%) ( Fig. 2.7).6 In the sellar type, the sphenoid sinus is pneumatized anterior and inferior to the sellar prominence. In the presellar type, the pneumatization is localized to the anterior sphenoid body and does not extend beyond the anterior sellar wall. A conchal sphenoid sinus has minimal to absent pneumatization. The sellar configuration is most favorable for transsphenoid surgery, whereas the conchal configuration is the most challenging anatomically. Lateral pneumatization into the sphenoid wing is most favorable for transpterygoid endoscopic approaches to the middle skull base.
Fig. 2.7).6 In the sellar type, the sphenoid sinus is pneumatized anterior and inferior to the sellar prominence. In the presellar type, the pneumatization is localized to the anterior sphenoid body and does not extend beyond the anterior sellar wall. A conchal sphenoid sinus has minimal to absent pneumatization. The sellar configuration is most favorable for transsphenoid surgery, whereas the conchal configuration is the most challenging anatomically. Lateral pneumatization into the sphenoid wing is most favorable for transpterygoid endoscopic approaches to the middle skull base.
Fig. 2.5 The left cavernous internal carotid artery is torturous with a dehiscent bony carotid canal (arrow), which makes it more vulnerable to injury during surgery.
Fig. 2.7 Variable pneumatization of the sphenoid sinus. In the conchal type (a), pneumatization does not extend into the sphenoid body. In the presellar type (b), pneumatization reaches but does not extend beyond the anterior sellar wall. In the sellar type (c,d), pneumatization extends posteriorly below the sella (incomplete sellar type, c), or all the way to the clival margin (complete sellar type, d). The sellar configuration is most favorable for transsphenoid surgery.
2.3 Tumors and Tumor-Like Conditions
One of the important roles of imaging in the evaluation of skull base neoplasia is to map the full extent of the disease. This is important in staging, which in turn will influence treatment approach and prognosis. Some imaging features important for surgical planning for central skull base tumors are listed in  Table 2.2. Documentation of the presence of some of these may severely limit or preclude the complete surgical excision of disease.
Table 2.2. Documentation of the presence of some of these may severely limit or preclude the complete surgical excision of disease.
2.3.1 Pituitary Adenoma
Pituitary adenomas are among the most common of all central nervous system (CNS) neoplasms. They are adenohypophysial tumors composed of secretory cells that produce pituitary hormones. The World Health Organization (WHO) classifies pituitary tumors as typical adenomas, atypical adenomas, and pituitary carcinomas. Pituitary adenomas are benign lesions, but both typical and atypical adenomas may have extensive invasion of the surrounding structures. Pituitary carcinomas are exceptionally rare and, by definition, have craniospinal dissemination or systematic metastases, although not all of them display classic cytological features of malignancy.7
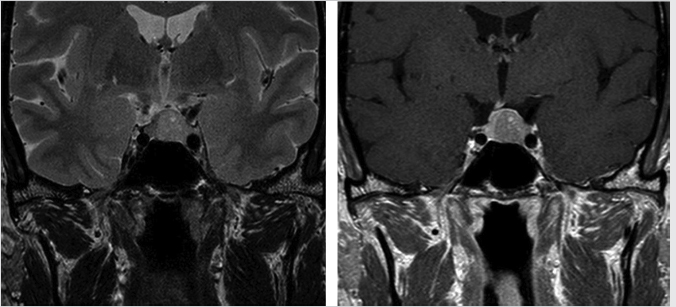
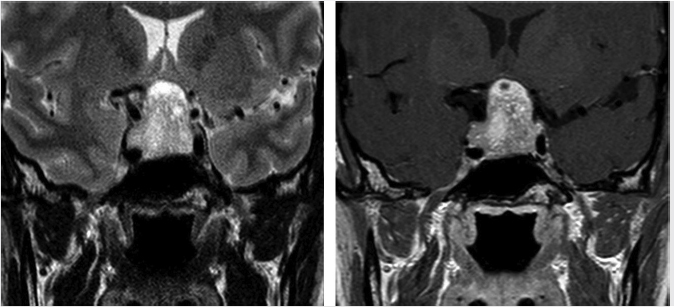
Microadenomas are defined as those lesions 10 mm or less in diameter ( Fig. 2.8), whereas macroadenomas refer to those greater than 10 mm (
Fig. 2.8), whereas macroadenomas refer to those greater than 10 mm ( Fig. 2.9,
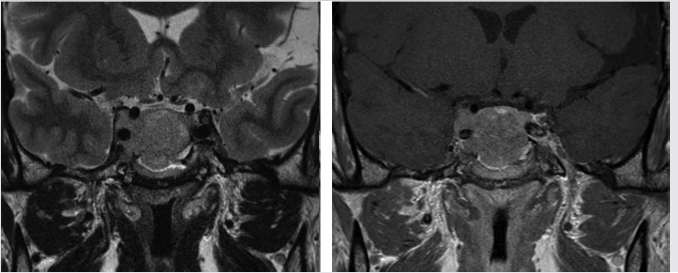
Fig. 2.9,  Fig. 2.10,
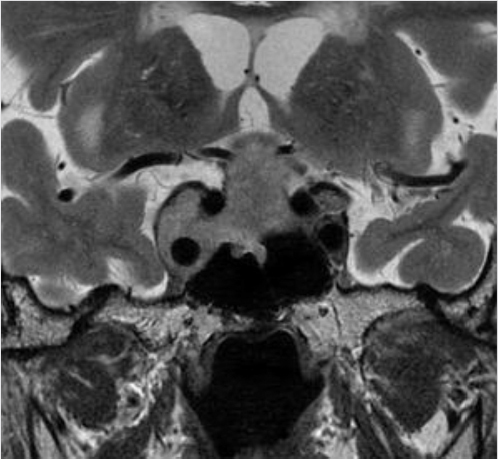
Fig. 2.10,  Fig. 2.11,
Fig. 2.11,  Fig. 2.12). Most pituitary adenomas occur in adults (peak age of presentation between the fourth and seventh decades) and are sporadic. Approximately 5% of pituitary adenomas are familial and occur in patients with multiple endocrine neoplasia (MEN) type 1, Carney complex, McCune-Albright syndrome, and familial isolated pituitary adenoma syndrome.
Fig. 2.12). Most pituitary adenomas occur in adults (peak age of presentation between the fourth and seventh decades) and are sporadic. Approximately 5% of pituitary adenomas are familial and occur in patients with multiple endocrine neoplasia (MEN) type 1, Carney complex, McCune-Albright syndrome, and familial isolated pituitary adenoma syndrome.
Approximately 75% of pituitary adenomas are hormone secreting, and 25% are nonfunctional.8 The clinical presentation of pituitary adenomas depends on the size of the lesion, hormonal activity, and the degree of extrasellar extension. Depending on the hormones being secreted, patients may present with amenorrhea, galactorrhea, hypogonadism, acromegaly, gigantism, Cushing’s disease, or hyperthyroidism. Nonfunctional adenomas generally present due to mass effect, most commonly headache and visual disturbances. Rarely, a pituitary adenoma may present acutely with pituitary apoplexy due to intratumoral hemorrhage.
Prolactinoma is the most common type of pituitary adenoma in clinical series, making up about 50% of patients presenting with endocrine disturbance. However, elevated prolactin levels are not always due to a hormonally active adenoma. Any process that interferes with the production, release, or pituitary portal venous transport of prolactin-inhibiting factors from the hypothalamus can result in hyperprolactinemia because of the resulting disinhibition of normal prolactin cells. Nevertheless, serum prolactin levels greater than 150 ng/mL (normal is < 20 ng/mL) are almost always due to a prolactinoma. This is less certain in patients with elevated serum prolactin less than 150 ng/mL.9
Table 2.2 Key imaging findings for surgical planning
1. Defining the epicenter and extent of tumor a) Intrasellar b) Suprasellar c) Clival |
2. Displacement of the pituitary gland, infundibulum, and optic apparatus by tumor should be noted, as should the location of the diaphragma sellae |
3. Describing the relationship to a) Internal carotid artery b) Optic nerve c) Optic chiasm d) Cavernous sinus |
4. Extent of lateral extension into the orbit, including status of a) Lamina papyracea b) Periorbita c) Orbital fat d) Orbital muscles e) Orbital apex |
5. Extent of intracranial extension a) Bony skull base erosion b) Dural involvement c) Intradural extension d) Relationship to ventricular system |
6. Degree of lateral extension into skull base a) Pterygopalatine fossa b) Infratemporal fossa c) Pterygoid plates d) Petrous apex e) Foramen magnum f) Occipital condyle g) Hypoglossal canal |
7. Presence and extent of perineural spread a) Vidian b) V1, 2, 3 c) Superior and inferior orbital fissures |
Clinically nonfunctional pituitary adenomas typically arise from gonadotroph cells. These cells make the alpha and beta subunits of the gonadotrophin hormones but not the intact molecule, and hence these lesions are not biologically active.
Imaging
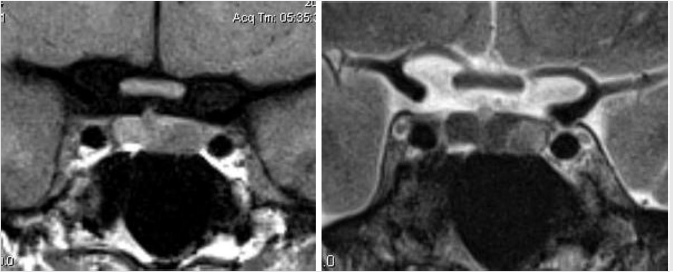
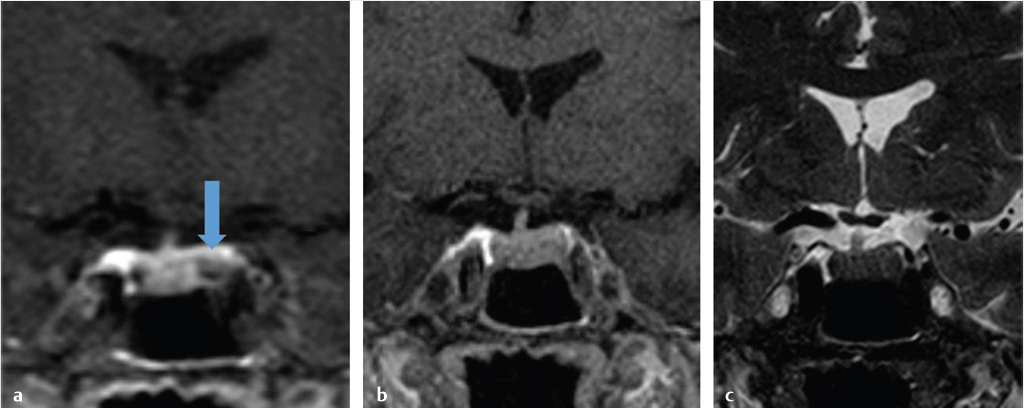
Dedicated magnetic resonance imaging (MRI) of the sella is the imaging modality of choice for assessing suspected pituitary abnormality. Although most adenomas are visible without intravenous contrast, several studies have shown improved visibility of small lesions with gadolinium.10,11,12,13,14,15,16 Furthermore, dynamic contrast-enhanced MRI of the sella can be done to further increase detection sensitivity, taking advantage of the differential rates of contrast enhancement between adenomas and normal pituitary gland ( Fig. 2.13).17,18,19,20 This increased sensitivity is most helpful in the detection and localization of adrenocorticotropic hormone (ACTH)-secreting adenomas in Cushing’s disease because these tumors tend to be small. Accurate documentation of the presence and position of these adenomas is important because surgical removal is the most effective treatment.
Fig. 2.13).17,18,19,20 This increased sensitivity is most helpful in the detection and localization of adrenocorticotropic hormone (ACTH)-secreting adenomas in Cushing’s disease because these tumors tend to be small. Accurate documentation of the presence and position of these adenomas is important because surgical removal is the most effective treatment.
A sellar or combined sellar and suprasellar mass that cannot be identified separately from the pituitary gland is the most characteristic imaging finding. An enlarged and remodeled sella turcia is commonly seen with macroadenomas. Inflammatory disorders (such as hypophysitis or sarcoidosis) can sometimes cause enlargement of the pituitary gland, which is often associated with enhancement of the adjacent meninges (a finding that is rare in adenomas).
Most pituitary adenomas are hypointense on T1-weighted images (T1WIs) and iso- to hyperintense on T2-weighted images (T2WIs) relative to normal pituitary tissue ( Fig. 2.8). Hemorrhagic and cystic areas may be present within the lesion. During dynamic imaging, pituitary adenomas tend to show slower enhancement than the rest of the gland.
Fig. 2.8). Hemorrhagic and cystic areas may be present within the lesion. During dynamic imaging, pituitary adenomas tend to show slower enhancement than the rest of the gland.
Fig. 2.8 Pituitary microadenoma. Most pituitary adenomas are hypointense on T1-weighted images and iso- to hyperintense on T2-weighted images relative to normal pituitary tissue.
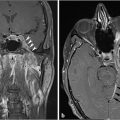
Fig. 2.9 Pituitary macroadenoma. Cavernous sinus invasion is possible in this Knosp grade 2 lesion, which extends to the median intercarotid line on the left side. The normal pituitary gland is displaced to the right.
Fig. 2.11 Pituitary macroadenoma. The lesion completely encases the right cavernous internal carotid artery (Knosp grade 4) and invades the right cavernous sinus.
Fig. 2.12 Large pituitary macroadenoma with right cavernous sinus invasion, completely encasing the right cavernous internal carotid artery (Knosp grade 4). The lesion compresses and elevates the optic chiasm.
No imaging feature definitively distinguishes between the various types of pituitary adenomas, although certain findings are more commonly seen with some. ACTH-secreting microadenomas are generally the smallest (mean size: 3 mm). Hormonally active adenomas have a certain topographic predilection within the gland that parallels the distribution of the normal secretory cells. Therefore, prolactin and growth hormone (GH) microadenomas tend to be lateral, whereas ACTH, thyroidstimulating hormone (TSH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) microadenomas tend to be central in location. GH-secreting adenomas tend to show infrasellar rather than suprasellar extension. GH-secreting adenomas are also more commonly T2 hypointense, a finding predictive of a densely granulated appearance on histopathology ( Fig. 2.14).21
Fig. 2.14).21
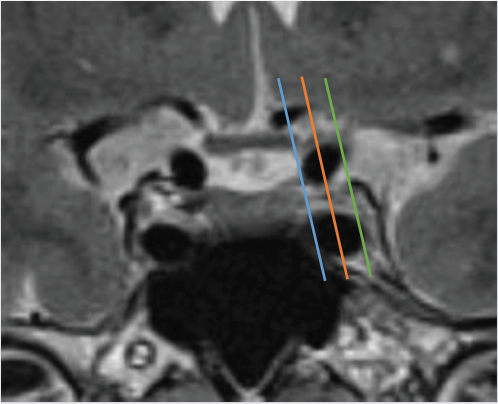
Lateral extension of the adenoma into the cavernous sinus impacts the surgeon’s ability to completely resect the tumor. However, differentiation between compression and invasion of the cavernous sinus on MRI is not always easy because the medial wall is very thin. Various parameters have been described in an effort to predict cavernous sinus invasion. The Knosp-Steiner grade of parasellar extension is based on the relationship between the tumor and tangential lines drawn between the cavernous and supraclinoid internal carotid arteries on coronal MR images ( Fig. 2.15).22 Knosp grades 3 and 4 (tumor extending beyond the lateral intercarotid line or totally encasing the cavernous carotid artery) are highly predictive of cavernous sinus invasion (
Fig. 2.15).22 Knosp grades 3 and 4 (tumor extending beyond the lateral intercarotid line or totally encasing the cavernous carotid artery) are highly predictive of cavernous sinus invasion ( Fig. 2.10,
Fig. 2.10,  Fig. 2.11,
Fig. 2.11,  Fig. 2.12). Knosp grade 0 and 1 lesions do not invade the cavernous sinus. Knosp grade 2 lesions sometimes show invasion (
Fig. 2.12). Knosp grade 0 and 1 lesions do not invade the cavernous sinus. Knosp grade 2 lesions sometimes show invasion ( Fig. 2.9).
Fig. 2.9).
Fig. 2.13 This pituitary microadenoma (arrow) is well seen on early dynamic contrast-enhanced image (a), but is difficult to appreciate on delayed contrast-enhanced (b) or T2-weighted images (c).
Fig. 2.14 Growth hormone (GH)-secreting pituitary adenoma. Note this lesion is hypointense on T2-weighted images, which is a finding seen more commonly in GH-secreting adenomas, particularly those that are densely granulated.
Treatment
The initial treatment of pituitary adenomas varies depending on tumor type and configuration. Adenomas that produce prolactin, GH, ACTH, or TSH can be treated with different medications initially or after surgery. Dopamine agonists have become the initial treatment for most patients with prolactinoma. Surgery is usually the first-line treatment option for GH and ACTH-producing adenomas and nonfunctioning adenomas.7
Different surgical options include transcranial, microsurgical, transsphenoidal, or pure endoscopic procedures. In recent years, the transsphenoid route has become the standard approach for most intrasellar and some suprasellar tumors because of lower morbidity and mortality rates when compared with transcranial procedures.
Radiation is usually considered when initial treatment fails, when surgical excision is incomplete, or when there is tumor recurrence. The two major types of radiation therapy are stereotactic radiosurgery and fractionated radiation therapy. Stereotactic radiosurgery can be delivered as a single treatment and has the advantage of reduced radiation exposure to the brain, and is well suited for smaller adenomas less than 30 mm that do not abut the optic structures or infiltrate into the surrounding structures. Larger adenomas are more safely treated with fractionated radiation due to the mitigating effect of using smaller doses per fraction in damaging late responding normal tissues such as brain and optic nerves.23
Fig. 2.15 Knosp grading of parasellar extension, based on the relationship of the pituitary tumor and tangential lines drawn between the cavernous supraclinoid internal carotid arteries on coronal MRI images. Blue line: medial intercarotid line connecting the medial walls of the internal carotid arteries (ICAs). Orange line: median intercarotid line connecting the centers of the ICAs. Green line: lateral intercarotid line connecting the lateral walls of the ICAs. Knosp grade 0 (tumor medial to the medial line) and grade 1 (tumor reaching the median line but not extending beyond it) are unlikely to have cavernous sinus invasion. Knosp grade 2 (tumor extending between the median and the lateral line) lesions sometimes have cavernous sinus invasion. Knosp grades 3 and 4 (tumor extending beyond the lateral intercarotid line or totally encasing the cavernous carotid artery) are highly predictive of cavernous sinus invasion.
Pituitary adenoma
Characteristic imaging features:
• Sellar or combined sellar and suprasellar mass that cannot be identified separately from the pituitary gland.
• Remodeling/enlargement of the sella turcica for macroadenomas.
Important imaging findings for treatment planning:
• Relationship to optic chiasm and optic nerves.
• Cavernous sinus extension.
• Internal carotid artery encasement.
• Effect on adjacent brain.
• Bony skull base invasion.
2.3.2 Craniopharyngioma
Craniopharyngiomas are rare and mostly benign tumors that arise from epithelial remnants of Rathke’s pouch that occur exclusively in the region of the sella turcica and suprasellar cistern or in the third ventricle. There is a bimodal age distribution, with more than half occurring in children between 5 and 14 years of age, and a second smaller peak in adults between 50 and 75 years of age.24
Craniopharyngiomas primarily occur in the suprasellar cistern. Infrequently, the lesions may be completely intrasellar or located in the third ventricle. Secondary to their anatomic location, craniopharyngiomas may present with endocrine dysfunction (in 80–90%), visual disturbance, and signs of increased intracranial pressure.
Two histological subtypes exist—adamantinomatous (more common) and papillary (less common, usually in adults). In addition to being histologically disparate, the two subtypes have different molecular genetics and characteristic imaging features.
Imaging
Adamantinomatous craniopharyngioma
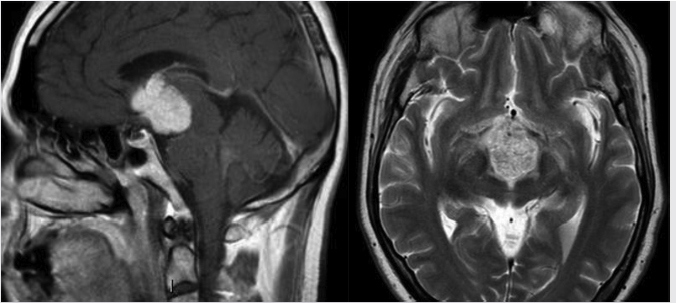
Adamantinomatous craniopharyngiomas are the more frequently encountered subtype, especially in the pediatric population. A partially calcified, mixed solid and cystic suprasellar lesion is the classic appearance ( Fig. 2.16). Approximately 90% contain calcification. Signal on MR varies with cyst content, and cyst signal can range from hypointense to hyperintense compared to brain on T1WI and T2WI.
Fig. 2.16). Approximately 90% contain calcification. Signal on MR varies with cyst content, and cyst signal can range from hypointense to hyperintense compared to brain on T1WI and T2WI.
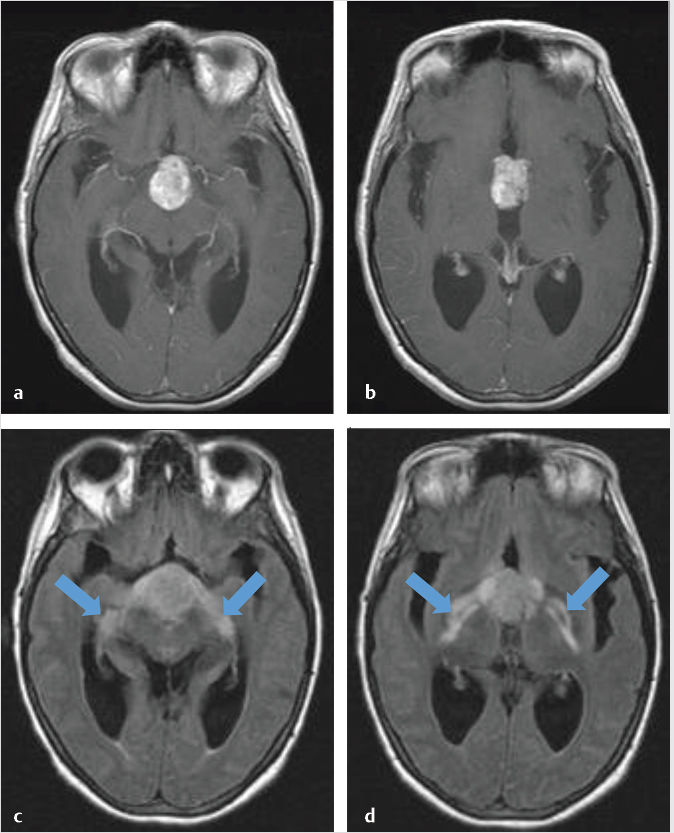
T2 hyperintensity along the optic tracts is a common finding and usually represents edema rather than tumor invasion ( Fig. 2.17). This finding, rather than optic nerve signal abnormality, may suggest a craniopharyngioma over other parasellar lesions,25 although it has reported in many other pathologies.26,27
Fig. 2.17). This finding, rather than optic nerve signal abnormality, may suggest a craniopharyngioma over other parasellar lesions,25 although it has reported in many other pathologies.26,27
Although craniopharyngiomas are grossly well circumscribed, microscopically the borders are frequently irregular and may be associated with gliosis in the adjacent brain tissue, which may be easily mistaken for glioma if biopsied.28 This reactive gliosis also results in an indistinct and adherent interface between the craniopharyngioma and adjacent brain, often making identification and manipulation of surgical planes difficult.
Papillary craniopharyngioma
Papillary craniopharyngiomas are usually found in adult patients. In distinction from their adamantinomatous counterpart, they are typically solid, without calcification, and are often found within the third ventricle ( Fig. 2.18). They have a nonspecific signal intensity pattern of a solid enhancing tumor. They are usually encapsulated and are readily separable from nearby structures, so recurrence rates are generally thought to be much less than within the adamantinomatous type.
Fig. 2.18). They have a nonspecific signal intensity pattern of a solid enhancing tumor. They are usually encapsulated and are readily separable from nearby structures, so recurrence rates are generally thought to be much less than within the adamantinomatous type.
Treatment
Surgical resection is the primary treatment for craniopharyngiomas. For several decades, transcranial approaches have been used widely and successfully. In recent years, the evolution of endoscopic endonasal surgery has opened new surgical alternatives, with several authors showing safety and effectiveness of this approach, with arguably better surgical advantages and superior outcomes.29
Radiation therapy is indicated in patients with residual disease who have undergone a partial surgical resection or to treat disease that has recurred following initial gross total resection. It may also be used in patients who are not surgical candidates. Excellent long-term tumor control has been reported in radiotherapy series.
Fig. 2.17 Axial postgadolinium T1-weighted (a,b) and FLAIR (c,d) images of a craniopharyngioma showing edema along the optic tracts (arrows; c,d).
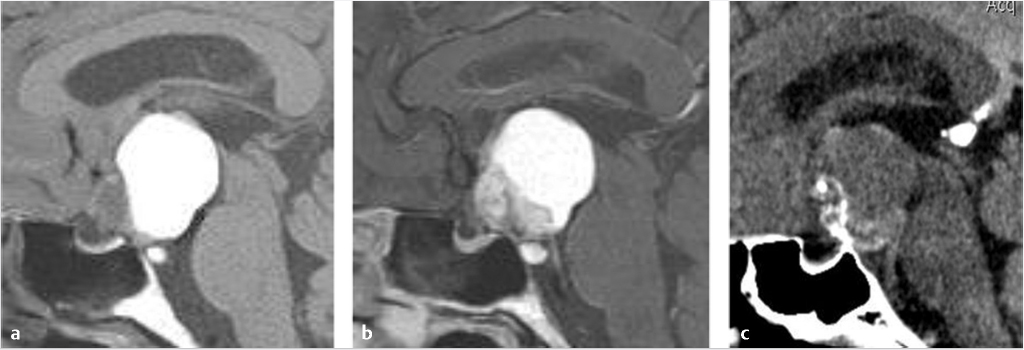
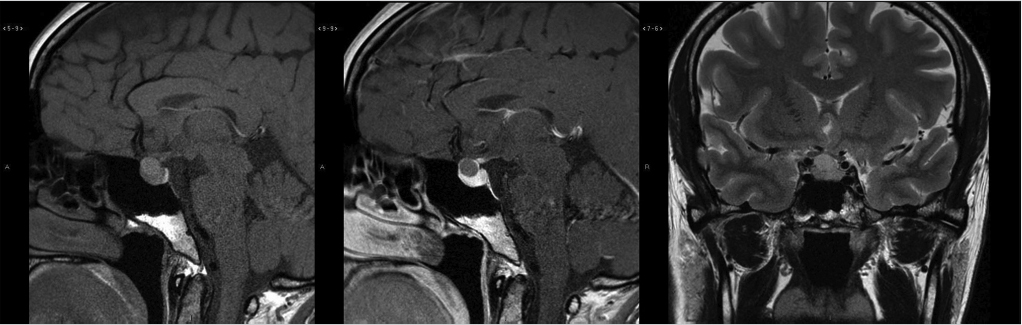
Fig. 2.19 Rathke’s cleft cyst. Cystic suprasellar mass with mild intrinsic T1 hyperintensity and mild peripheral enhancement.
Craniopharyngioma
Characteristic imaging features:
• Multilobulated complex mass with cystic and solid components, usually centered in the suprasellar region.
• The adamantinomatous subtype is more common, especially in children.
• The papillary subtype is less common and is usually seen in adults, and is characteristically solid.
• T2 hyperintensity along the optic tracts is suggestive.
• Can be adherent to adjacent brain, which can show signal alteration due to secondary gliosis.
Important imaging findings for treatment planning:
• Relationship to the optic apparatus and adjacent arteries.
• Effect on adjacent brain.
2.3.3 Rathke’s Cleft Cyst
Rathke’s cleft cysts are thought to arise from remnants of the fetal Rathke’s pouch. They are a common incidental finding at autopsy (about 11% in nonselected specimens).30 They are predominantly intrasellar in location. Others may be centered in the suprasellar cistern, usually midline and just anterior to the pituitary stalk. Most Rathke’s cleft cysts are small and asymptomatic, but symptoms can occur if it is large enough to compress the pituitary gland or optic chiasm, or in rare instances of hemorrhage. Most Rathke’s cleft cysts remain stable or grow slowly over time.
Imaging
Rathke’s cleft cysts appear as a well-defined round or ovoid mass within or just above the sella turcica ( Fig. 2.19). The internal content varies from hypointense to hyperintense on T1WI, and is usually hyperintense on T2WI and fluid attenuation inversion recovery (FLAIR). An intracystic nodule can be present, which usually consists of mucin clump and is T1 hyperintense.31,32 Calcification is uncommon. They do not typically enhance, although occasional thin marginal enhancement of the cyst wall has been observed.
Fig. 2.19). The internal content varies from hypointense to hyperintense on T1WI, and is usually hyperintense on T2WI and fluid attenuation inversion recovery (FLAIR). An intracystic nodule can be present, which usually consists of mucin clump and is T1 hyperintense.31,32 Calcification is uncommon. They do not typically enhance, although occasional thin marginal enhancement of the cyst wall has been observed.
Treatment
No treatment is necessary for asymptomatic Rathke’s cleft cysts. Partial resection or aspiration is usually sufficient for symptomatic lesions, usually via transsphenoidal surgery. Recurrence rates after surgery range between 16 and 18% in large series, with higher rates associated with suprasellar location, inflammation and reactive squamous metaplasia in the cyst wall, superinfection of the cyst, and placement of a fat graft into the cyst cavity.33
Characteristic imaging features:
• Well-defined round mass within or just above the sella turcica.
• T1 hyperintense intracystic nodule can be present and is suggestive.
• Lack of calcification may help distinguish from craniopharyngioma.
2.3.4 Meningioma
Approximately 10% of meningiomas occur in the parasellar region,34 arising from a variety of locations including the tuberculum sellae, clinoid processes, medial sphenoid wing, and cavernous sinus. Meningiomas can also extend into the parasellar region from other sites, such as a planum sphenoidale meningioma extending posteriorly into the suprasellar cistern or sella turcica.
Meningiomas occur most frequently in the sixth decade of life and have a female predilection. Most are sporadic. Patients with neurofibromatosis type 2 and prior cranial radiation have an increased incidence. Meningiomas are usually slow-growing lesions that may be found incidentally or present because of compression, or less commonly invasion, of adjacent structures.
Most meningiomas are histologically typical (WHO grade 1), but about 4 to 8% are classified histologically as atypical meningiomas (WHO grade 2) and 1 to 3% are classified as malignant meningiomas (WHO grade 3).35 The skull base is a relatively rare location for these more aggressive subtypes. In general, it can be difficult to determine the tumor grade based on imaging. Atypical and malignant meningiomas can have indistinct borders with the adjacent parenchymal cortex, and peritumoral edema is a common but nonspecific finding.
Imaging
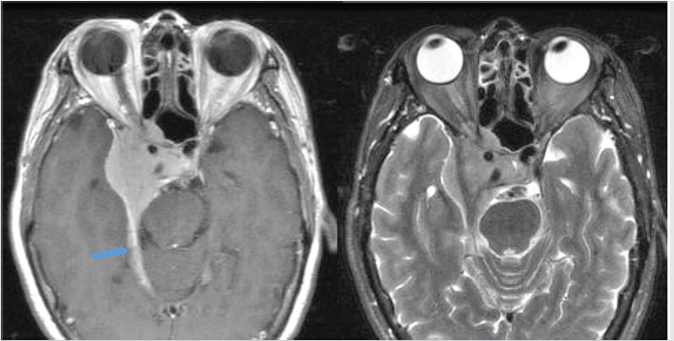
Most meningiomas are hyperdense compared to brain parenchyma on CT ( Fig. 2.20), and about 25% show calcification. On MR, they are usually hypointense to isointense relative to gray matter on T1WI, and isointense to moderately hyperintense on T2WI. Virtually all meningiomas enhance, usually strongly and homogenously. A dural tail is seen in the majority of meningiomas (
Fig. 2.20), and about 25% show calcification. On MR, they are usually hypointense to isointense relative to gray matter on T1WI, and isointense to moderately hyperintense on T2WI. Virtually all meningiomas enhance, usually strongly and homogenously. A dural tail is seen in the majority of meningiomas ( Fig. 2.21). Hyperostosis in the adjacent bone, best appreciated on CT, can be present and is a characteristic feature (
Fig. 2.21). Hyperostosis in the adjacent bone, best appreciated on CT, can be present and is a characteristic feature ( Fig. 2.20,
Fig. 2.20,  Fig. 2.22). Necrosis, cyst, hemorrhage, or fat can be seen within meningiomas but are infrequently seen. Peritumoral cyst and edema within the adjacent brain are sometimes present (
Fig. 2.22). Necrosis, cyst, hemorrhage, or fat can be seen within meningiomas but are infrequently seen. Peritumoral cyst and edema within the adjacent brain are sometimes present ( Fig. 2.20).
Fig. 2.20).
Vascular encasement is not uncommon, particularly for tumors in the cavernous sinus. Meningiomas characteristically constrict the lumen of the encased vessel ( Fig. 2.23), in contrast with pituitary adenoma for which this is rare. However, luminal narrowing can be seen in other entities such as metastasis, chordoma, lymphoma, or Tolosa-Hunt. Some imaging features that are suggestive of atypical (WHO grade 2) and malignant (WHO grade 3) meningiomas include indistinct borders of the mass with the adjacent cortex, bony infiltration, and more pronounced peritumoral edema (
Fig. 2.23), in contrast with pituitary adenoma for which this is rare. However, luminal narrowing can be seen in other entities such as metastasis, chordoma, lymphoma, or Tolosa-Hunt. Some imaging features that are suggestive of atypical (WHO grade 2) and malignant (WHO grade 3) meningiomas include indistinct borders of the mass with the adjacent cortex, bony infiltration, and more pronounced peritumoral edema ( Fig. 2.24). WHO grade 2 and 3 meningiomas will also tend to show a greater degree of restricted diffusion which reflects their greater cellularity.
Fig. 2.24). WHO grade 2 and 3 meningiomas will also tend to show a greater degree of restricted diffusion which reflects their greater cellularity.
Fig. 2.20 Meningiomas tend to be hyperintense relative to brain on CT. Hyperostosis in the adjacent bone (arrow) is another characteristic feature.
Fig. 2.21 Meningioma centered in the right parasellar region extending into the right orbital apex and encasing the right internal carotid artery. Note the dural tail (arrow) and relatively low T2 signal.
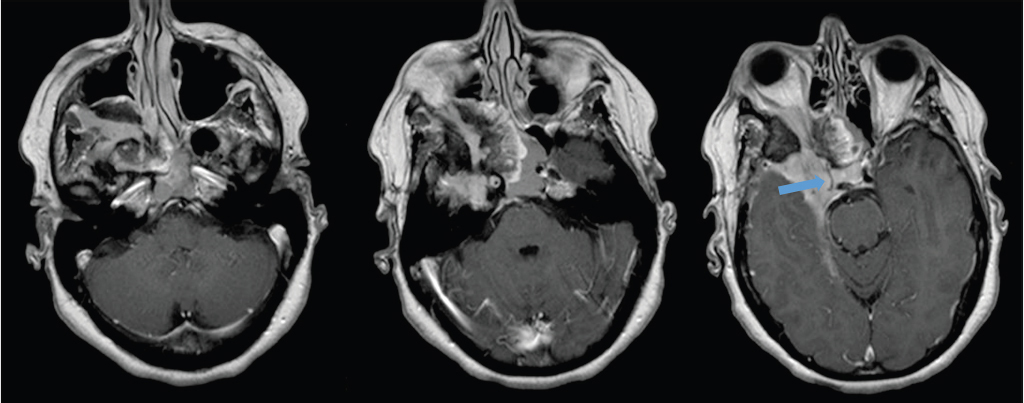
Fig. 2.23 Extensive skull base meningioma involving the right middle cranial fossa and parasellar region, extending through multiple skull base foramen (superior orbital fissure, inferior orbital fissure, foramen rotundum), with tumor in the right orbital apex and retroantral space. The cavernous portion of the right internal carotid artery is encased by the tumor and markedly narrowed (arrow).
Treatment
Elderly patients with small asymptomatic meningiomas may be observed and treatment initiated if and when there is progression. Surgery is most likely to achieve cure; however, the likelihood of accomplishing a complete resection is related to the location and extent of the tumor. Skull base meningiomas are particularly challenging because they often cannot be completely resected, and there may be significant surgical morbidity due to the complex anatomy with many nearby critical neural and vascular structures ( Fig. 2.25,
Fig. 2.25,  Fig. 2.26). Radiotherapy and radiosurgery are alternative treatment methods with similar long-term local control and survival rates compared to surgery.36 Radiation can be used primarily or adjunctive when resection is incomplete. In general, radiosurgery is indicated if the lesion is small enough to be encompassed in the field, whereas larger, irregular tumors are treated with fractionated radiotherapy.
Fig. 2.26). Radiotherapy and radiosurgery are alternative treatment methods with similar long-term local control and survival rates compared to surgery.36 Radiation can be used primarily or adjunctive when resection is incomplete. In general, radiosurgery is indicated if the lesion is small enough to be encompassed in the field, whereas larger, irregular tumors are treated with fractionated radiotherapy.
Meningioma
Characteristic imaging features:
• Dural-based enhancing mass may contain calcification.
• Tends to narrow vessel when there is encasement.
• Atypical and malignant meningiomas are rare in the skull base. They tend to have an indistinct border with the adjacent cortex and peritumoral edema is more commonly seen.
Important imaging findings for treatment planning:
• Relationship with the optic apparatus, extension into the optic canal and orbital apex.
• Relationship with the adjacent arteries (displacement, encasement, and luminal narrowing).
• Relationship with cranial nerves and skull base foramina.
• Relationship with the pituitary gland and stalk.
• Relationship with the cavernous sinuses.
• Effect on adjacent brain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree